TB is still an important problem all over the world but latent TB testing is currently known to be an effective prevention area. The region of North America particularly the United States of America and Canada has provided a model on how to embrace modern technology and policies in the elimination of LTBI. Latent TB testing has put this region among the world’s favorites because of its well-developed healthcare, and regulatory policies and strategies. In this article, the author examines how North America has adopted latent TB testing, and what its vision of the market is.
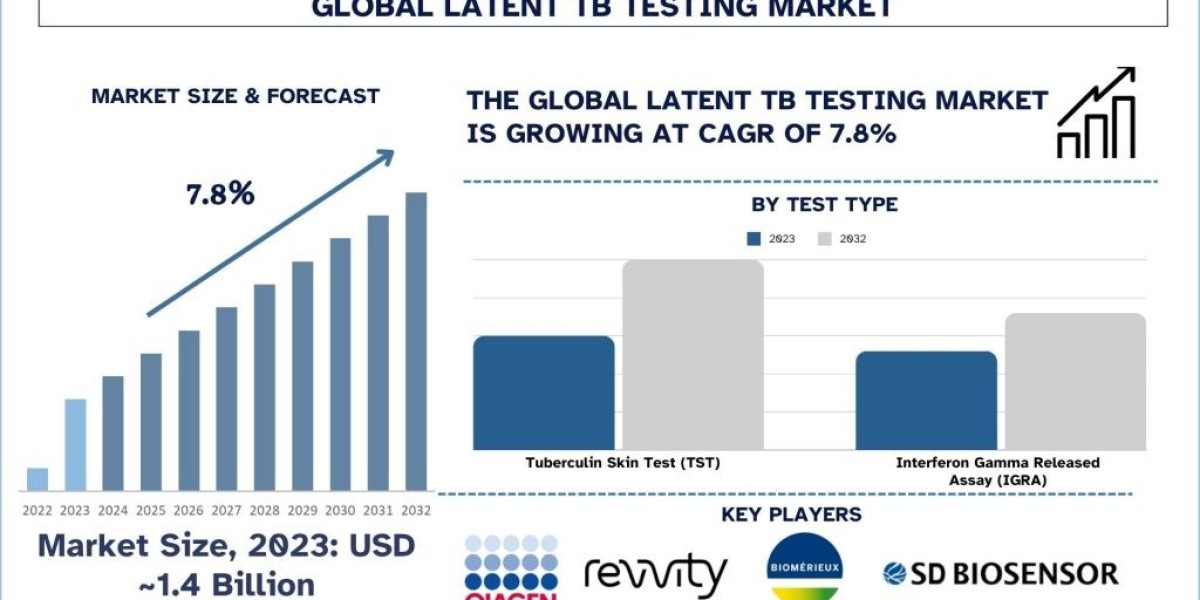
According to the UnivDatos Market Insights, rising tuberculosis (TB) incidence, increased need for screening in HIV/AIDS and transplant patients, rising advanced diagnostics, and adoption of IGRAs drive the Latent TB Testing market. As per their “Latent TB Testing Market” report, the global market was valued at USD 1.4 Billion in 2023, growing at a CAGR of about 7.8% during the forecast period from 2024 - 2032 to reach USD Billion by 2032.
For More Detailed Analysis in PDF Format, Visit- https://univdatos.com/get-a-free-sample-form-php/?product_id=68927
The Latent TB Testing Market in North America Report
North America has remained the leading market for latent TB testing due to the strong regulatory policies adopted, coupled with the availability of complex diagnostic tools. According to the latest data, the trend in TB rates is still low compared to average values for the world, however, targeted screening for latent TB is needed to avoid sporadic outbreaks. Target groups include immigrants from TB-endemic countries, healthcare providers, and persons with immunosuppression that make up the core of testing.
On 24 March 2021, World Tuberculosis (TB) Day and bioMérieux, a world leader in the field of in vitro diagnostics, announced the CE marking of its innovative and fully-automated test VIDAS® TB IGRA (Interferon-Gamma Release Assay) to diagnose latent TB infection.
Currently, the market mainly consists of new diagnostic techniques, such as IGRAs that are considered to be more accurate than TSTs. Government sponsorship and partnerships with industry players have gone further to drive the use of these tests hence making North America a reference region.
Adoption Strategies of the U.S. and Canada
United States: Pioneering Precision Diagnostics
The United States has embraced latent TB testing through accuracy and expansion making it the forerunner. The regulations include those of the CDC and state health departments that require latent TB screening of the at-risk population including immigrants, healthcare personnel, and inmates. Currently, the frequent application of IGRAs such as QuantiFERON-TB Gold Plus and T-SPOT.TB is explained by their capacity for giving reliable outcomes, including where patients have been BCG-vaccinated.
On March 23, 2023, PerkinElmer’s Oxford Immunotec announced that the U.S. Food and Drug Administration (FDA) has approved the use of two additional cell isolation instruments with the Company’s previously approved T-Cell Select™ reagent kit, which is intended for in vitro diagnostic (IVD) use by certified laboratories with the T-SPOT®.TB test workflow.
Market development has been greatly aided by the involvement of the private sector. Due to the adaptation of technology in the current generation, organizations are utilizing automation and digital solutions to incorporate LTBI into usual diagnostics. Such efforts are financed by federal grants through TB elimination programs which include funding for any proposed community screening and treatment programs.
Canada: Community-Centric Approaches
The Canadian strategy focuses on equality and participation of the community. Latent TB testing is a priority under the country’s Public Health Agency; TB screening is included in immigration policies and Indigenous health services. This obsession with cost-effectiveness has led to the promotion of low-price TSTs and a slow, but steady uptake of IGRAs in the large urban population.
Challenges in the Region
However, North America has some issues related to the detection of latent TB. IGRAs are expensive to the extent that they remain a preserve of the uninsured or low-income earners due to numerous cost barriers. Lack of knowledge in some groups increases testing because not many people come forward for testing, especially in rural areas. Also worthy of note are other factors such as accessibility of health facilities in rural areas other factors of transport, and cultural barriers to the stigmatization of TB patients.
Trend: The move to a sustainable future is the biggest trend that organizations will have to recognize in the future days Since its emergence this transnational company has had to recognize many trends as a way of carrying out its business but the most important trend that organizations will have to understand in future days is the transition towards a sustainable future.
Integration with Digital Health
Based on the findings of this study and using the knowledge available today, the future of latent TB testing in North America relies on digital health. Labs and diagnostics firms are now looking at deploying more such conventional systems to interpret results as well as future probable latent to active TB transition. This is also anticipated to be achieved through the utilization of telemedicine services to extend screening or else treatment into high geographical risk areas.
Focus on Home-Based Testing
Technological advancements to home-based latent TB testing kits will probably offer a strategic point, and enhance screening compliance. These kits are presumed to have simple technologies for the efficiency of the results delivered.
Expansion of IGRAs
The use of IGRAs is also expected to increase as diagnostic companies strive to develop ways of making the tests cheaper and more portable. This shift will enable the early replacement of established TSTs especially in high-risk populations including hospitals and prisons.
On March 18, 2024, QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) announced a partnership with the International Panel Physicians Association (IPPA) to support and educate panel physicians around the globe on the latest tuberculosis (TB) screening requirements. A special focus will be on the new Interferon Gamma Release Assay (IGRA) requirements and the associated benefits for patients and healthcare providers.
Improving Publicity on Health Careers
Stepping up public awareness of the potential danger of latent TB remains an important issue. Future campaigns will likely be aimed at ensuring that susceptible persons and the workforce are tested and treated as early as possible.
The following Sustainability in Testing Solutions was prepared by the writer to reflect on some of the cardinal aspirations of IT and Information Science for diverse testing solutions:
The topic of sustainability is emerging, and organizations are introducing environmentally friendly diagnostic instruments and decreasing the environmental footprint of testing processes.
Explore the Comprehensive Research Overview - https://univdatos.com/report/latent-tb-testing-market
Browse Related Trending Reports of UnivDatos Market Insights:
Exterior Insulation and Finish System (EIFS) Market: Current Analysis and Forecast (2024-2032)
Modular Bathroom Pods Market: Current Analysis and Forecast (2024-2032)
GLP-1 Analogues Market: Current Analysis and Forecast (2024-2032)
Train Toilets Market: Current Analysis and Forecast (2024-2032)
Nanocoatings Market: Current Analysis and Forecast (2024-2032)
Conclusion
The latent TB testing market of North America was one of the most active regions around the world when it came to adopting and implementing new measures for TB prevention. The region has improved the diagnostic capacity and management of LTBIs using improved technology, supportive policies, and collaborations.
However, depending on the 4P model, there may be following changes shortly, the introduction of digital health tools, home-based testing, and more awareness programs in the market. Indeed, by building infrastructures, overcoming existing difficulties, and strengthening the healthcare systems, North America has the preconditions to exercise leadership in the fight against TB and contribute to meeting the set goals of the elimination of this disease.
Contact Us:
UnivDatos Market Insights
Contact Number - +19787330253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/