IMARC Group, a leading market research company, has recently released a report titled “Preclinical CRO Market Report by Service (Bioanalysis and DMPK Studies, Toxicology Testing, and Others), End Use (Biopharmaceutical Companies, Government and Academic Institutes, Medical Device Companies), and Region 2024-2032”. The study provides a detailed analysis of the industry, including the preclinical CRO market share, trends, size, and industry trends forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
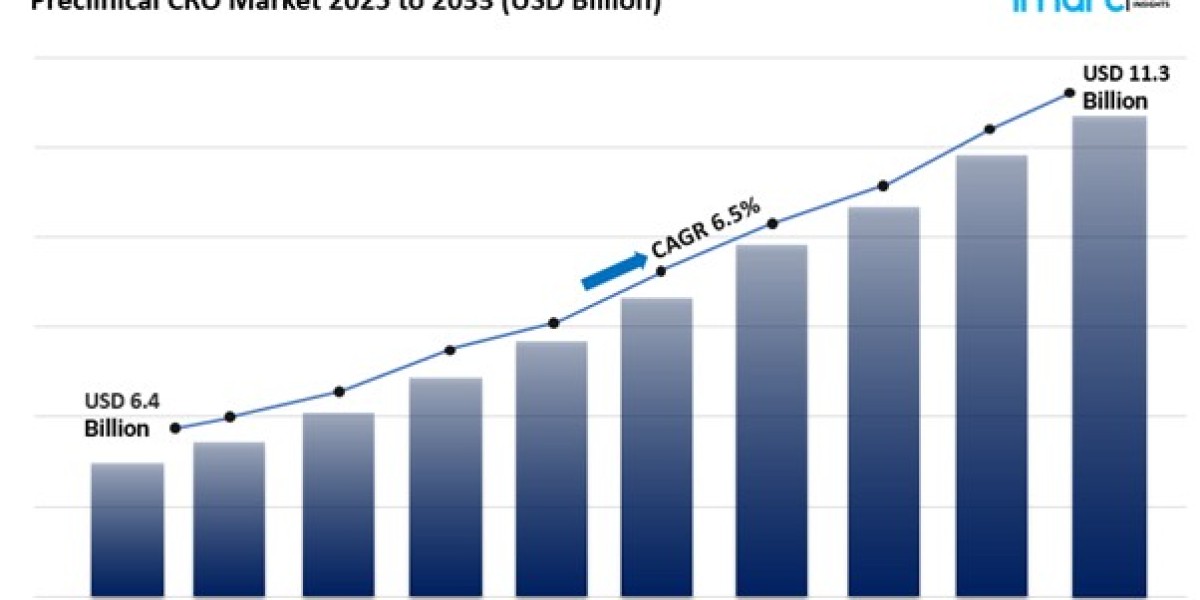
The global preclinical CRO market size reached USD 6.4 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 11.3 Billion by 2033, exhibiting a growth rate (CAGR) of 6.5% during 2025-2033.
Request to Get the Sample Report:
https://www.imarcgroup.com/preclinical-cro-market/requestsample
Preclinical CRO Market Trends in 2024
The preclinical CRO market is set for big changes next year. Factors like new technology and changing client needs are key. As drug firms focus on early research, demand for preclinical services will rise. This is especially true for areas like immunotherapy and gene therapy.
In 2024, the spotlight will be on personalized medicine. CROs will need to create models that match individual patient profiles. The use of AI and machine learning will boost data analysis and predictions. This leads to better decision-making.
Moreover, there will be a push for sustainable practices. CROs will adopt eco-friendly. methods to align with industry trends. In summary, 2024 will see innovation and teamwork in pharma and biotech. There will be a strong focus on clients' needs.
Market Dynamics of Preclinical CRO Market
Boost in Drug Development Funding
Pharmaceutical and biotech firms are now investing more in early drug development. This shift is due to the demand for new treatments for cancer, brain disorders, and rare diseases. With larger research budgets, companies are turning to specialized CROs for preclinical studies. This move taps into CROs' expertise and cuts costs. It also speeds up development and lets companies focus on what they do best.
In response, CROs are expanding their services. They now offer better testing models, toxicology studies, and drug absorption tests. CROs must meet strict regulations to stay competitive. So, they are upgrading their technology and facilities.
Advancements in technology and innovation.
New technologies are transforming preclinical CROs. They now demand more advanced methods. Innovations like high-throughput screening, organ-on-a-chip, and AI are key. These methods improve data accuracy and speed up drug development. They make decision-making quicker and cut development time.
Moreover, incorporating data analytics and bioinformatics helps find and predict drug candidates better. This is crucial for pharma companies. They now prefer CROs that use these advanced technologies. This gives those CROs a competitive edge. The trend shows no signs of slowing. CROs are investing in research to stay ahead and meet client needs.
Regulatory Pressure and Compliance
The preclinical CRO market is growing due to stricter drug development regulations. Agencies are enforcing tougher rules to ensure new therapies are safe and effective. This shift impacts preclinical studies significantly. So, pharmaceutical companies now seek CROs with regulatory expertise. These CROs help navigate compliance complexities. CROs are improving their GLP, documentation, and reporting due to rising demand.
Also, the need for data integrity and transparency is prompting them to adopt better quality management systems. As regulations change, a compliance and quality record will attract more clients and partners.
Preclinical CRO Market Report Segmentation:
By Service:
- Bioanalysis and DMPK Studies
- Toxicology Testing
- Others
Toxicology testing accounted for the largest market share as it is essential for ensuring the safety of drug candidates before clinical trials, making it a critical component of preclinical studies.
By End Use:
- Biopharmaceutical Companies
- Government and Academic Institutes
- Medical Device Companies
Biopharmaceutical companies represented the largest segment as they rely on preclinical CROs to streamline drug development processes and reduce costs.
Regional Insights:
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East and Africa
North America's dominance in the preclinical CRO market is attributed to its advanced healthcare infrastructure, high research, and development (R&D) spending, and the presence of numerous leading pharmaceutical and biotechnology companies.
Competitive Landscape with Key Players:
The competitive landscape of the preclinical CRO market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
- Charles River Laboratories Inc.
- Covance Inc. (Laboratory Corporation of America Holdings)
- Eurofins Scientific
- ICON Plc
- MD Biosciences Inc. (MLM Medical Labs)
- Medpace
- Parexel International Corporation
- PPD Inc.
- Wuxi AppTec
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=3715&flag=C
Key Highlights of the Report:
- Market Performance (2018-2023)
- Market Outlook (2024-2032)
- Market Trends
- Market Drivers and Success Factors
- Impact of COVID-19
- Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: sales@imarcgroup.com
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 | Asia: +91-120-433-0800