Key Market Insights
- Several service providers, across the globe, have the necessary capabilities to offer viral clearance and testing services for the detection and removal of various enveloped and non-enveloped viruses
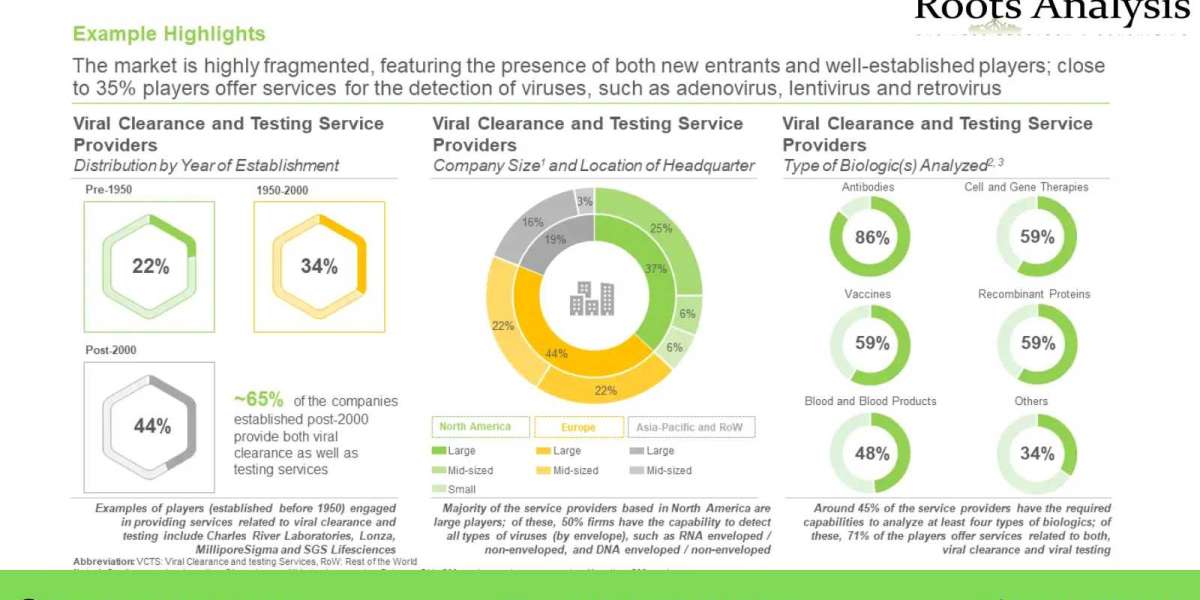
- The market is highly fragmented, featuring the presence of both new entrants and well-established players; close to 35% players offer services for the detection of viruses, such as adenovirus, lentivirus and retrovirus
- With around 75 facilities, viral clearance and testing service providers have established global presence; majority of these players are based in Europe, primarily in countries, such as the UK, Germany and France
- In pursuit of building a competitive edge, stakeholders are actively upgrading their existing capabilities and enhancing their respective service offerings to comply with the evolving industry benchmarks
- To protect the intellectual property generated within viral clearance and testing field, both industry and non-industry players have filed / were granted close to 260 patents in the past five years
- Over the past few years, several recent expansions and partnership activities have taken place in order to meet the increasing demand for viral clearance and testing services, worldwide
- The Viral Clearance and Testing Services market is projected to grow at a CAGR of ~10%, till 2035; the forecasted opportunity is likely to be distributed across different scales of operation, methods of viral clearance and testing, end users, and geographical regions
Table of Content
- PREFACE
1.1. Chapter Overview
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Viral Contamination in Biologics
3.3. Need for Viral Clearance and Testing
3.4. Process of Viral Clearance and Testing
3.5. Regulatory Guidelines Related to Viral Contamination
3.6. Future Perspectives
- VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Viral Clearance and Testing Service Providers: Overall Market Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Location of Headquarters
4.2.4. Analysis by Company Size and Location of Headquarters (Region)
4.2.5. Analysis by Location of Viral Clearance and Testing Facilities
4.2.6. Analysis by Type of Virus Detected (by Envelope)
4.2.7. Analysis by Type of Virus Detected (by Class)
4.2.8. Analysis by Key Offerings
4.2.9 Analysis by Type of Biologic(s) Analyzed
4.2.10 Analysis by Method(s) of Viral Clearance (Inactivation)
4.2.11 Analysis by Method(s) of Viral Clearance (Removal)
4.2.12 Analysis by Type of Viral Testing Service(s) Offered
- COMPANY COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2 Methodology and Key Parameters
5.3. Viral Clearance and Testing Service Providers in North America
5.4. Viral Clearance and Testing Service Providers in Europe
5.5. Viral Clearance and Testing Service Providers in Asia-Pacific and Rest of the World
- VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS IN NORTH AMERICA
6.1. Chapter Overview
6.2. Charles River Laboratories
6.2.1. Company Overview
6.2.2. Financial Information
6.2.3. Viral Clearance and Testing Related Services
6.2.4. Recent Developments and Future Outlook
6.3. Microbac Laboratories
6.3.1. Company Overview
6.3.2. Viral Clearance and Testing Related Services
6.3.3. Recent Developments and Future Outlook
6.4. Nelson Labs
6.4.1. Company Overview
6.4.2. Financial Information
6.4.3. Viral Clearance and Testing Related Services
6.4.4. Recent Developments and Future Outlook
6.5. Pall Corporation
6.5.1. Company Overview
6.5.2. Financial Information
6.5.3. Viral Clearance and Testing Related Services
6.5.4. Recent Developments and Future Outlook
- VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS IN EUROPE AND ASIA-PACIFIC
7.1. Chapter Overview
7.2. Eurofins Scientific
7.2.1. Company Overview
7.2.2. Financial Information
7.2.3. Viral Clearance and Testing Related Services
7.2.4. Recent Developments and Future Outlook
7.3. Syngene International
7.3.1. Company Overview
7.3.2. Financial Information
7.3.3. Viral Clearance and Testing Related Services
7.3.4. Recent Developments and Future Outlook
7.4. Texcell
7.4.1. Company Overview
7.4.2. Viral Clearance and Testing Related Services
7.4.3. Recent Developments and Future Outlook
- PATENT ANALYSIS
8.1. Chapter Overview
8.2. Scope and Methodology
8.3. Viral Clearance and Testing Services Market: Patent Analysis
8.3.1. Analysis by Publication Year
8.3.2. Analysis by Application Year
8.3.3. Analysis by Type of Patent and Publication Year
8.3.4. Analysis by Type of Applicant
8.3.5. Word Cloud Analysis: Emerging Focus Areas
8.3.6. Analysis by Patent Jurisdiction
8.3.7. Analysis by CPC Symbols
8.3.8. Leading Players: Analysis by Number of Patents
8.4. Viral Clearance and Testing Services Market: Patent Benchmarking Analysis
8.4.1. Analysis by Patent Characteristics
8.5. Viral Clearance and Testing Services: Patent Valuation Analysis
8.6. Leading Patents by Number of Citations
- RECENT DEVELOPMENTS
9.1 Chapter Overview
9.2. Partnerships and Collaborations
9.2.1. Partnership Models
9.2.2. Viral Clearance and Testing Service Providers: List of Partnerships and Collaborations
9.2.3. Analysis by Year of Partnership
9.2.4. Analysis by Type of Partnership
9.2.5. Analysis by Year and Type of Partnership
9.2.6. Analysis by Purpose of Partnership
9.2.7. Analysis by Type of Organization
9.2.8. Analysis by Location of Facility (Country)
9.2.9. Analysis by Location of Facility (Region)
9.2.10. Most Active Players: Analysis by Number of Partnerships
9.2.11. Analysis by Geography
9.2.11.1. Intercontinental and Intracontinental Agreements
9.3. Recent Expansions
9.3.1. Viral Clearance and Testing Service Providers: List of Recent Expansions
9.3.2. Analysis by Year of Expansion
9.3.3. Analysis by Type of Expansion
9.3.4. Analysis by Focus of Expansion
9.3.5. Analysis by Type of Service(s) Offered
9.3.6. Analysis by Type of Biologic(s) Involved
9.3.7. Analysis by Type of Expansion and Biologic(s) Involved
9.3.8. Most Active Players: Analysis by Number of Expansions
9.3.9. Analysis by Geography
- VIRAL CLEARANCE AND TESTING SERVICES: MARKET FORECAST AND OPPORTUNITY ANALYSIS
10.1. Chapter Overview
10.2. Forecast Methodology and Key Assumptions
10.3. Global Viral Clearance and Testing Services Market, 2023-2035
10.3.1. Global Viral Clearance and Testing Services Market: Distribution by Scale of Operation, 2023 and 2035
10.3.1.1. Viral Clearance and Testing Services Market for Discovery Phase, 2023-2035
10.3.1.2. Viral Clearance and Testing Services Market for Preclinical Phase, 2023-2035
10.3.1.3. Viral Clearance and Testing Services Market for Clinical Phase, 2023-2035
10.3.2. Global Viral Clearance and Testing Services Market: Distribution by Method of Viral Clearance and Testing, 2023 and 2035
10.3.2.1. Viral Clearance and Testing Services Market for Viral Detection, 2023-2035
10.3.2.2. Viral Clearance and Testing Services Market for Viral Inactivation, 2023-2035
10.3.2.3. Viral Clearance and Testing Services Market for Viral Removal, 2023-2035
10.3.3. Global Viral Clearance and Testing Services Market: Distribution by End-User, 2023 and 2035
10.3.3.1. Viral Clearance and Testing Services Market for Biotechnology and Pharmaceutical Companies, 2023-2035
10.3.3.2. Viral Clearance and Testing Services Market for Academic / Research Institutes, 2023-2035
10.3.4. Global Viral Clearance and Testing Services Market: Distribution by Key Geographical Regions, 2023 and 2035
10.3.4.1. Viral Clearance and Testing Services Market in North America, 2023-2035
10.3.4.2. Viral Clearance and Testing Services Market in Europe, 2023-2035
10.3.4.3. Viral Clearance and Testing Services Market in Asia-Pacific, 2023-2035
10.3.4.4. Viral Clearance and Testing Services Market in Rest of the World, 2023-2035
- CONCLUSION
- EXECUTIVE INSIGHTS
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/viral-clearance-and-testing-services-market.html
You may also be interested in the following titles:
DNA and Gene Cloning Services Market
Lipid Nanoparticles in Drug Delivery
You may also like to learn what our experts are sharing in Roots educational series:
Long-Acting Drug Delivery: A Novel Pharmacological Strategy to Deliver Therapeutic Modalities |
Digital Therapies: The “Digital Pills” of current generation |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415