Documents Required For Wholesale Drug License
Drug License
To start the wholesale Pharma business, a drug license is required. State Licensing Authority (SLA) issue the drug license in India.
Normally, the State Licensing Authority issues two types of Drug licenses - one is a Retail drug license which is issued to a person who runs a chemist shop sells medicine to retail consumers, and another one is a Wholesale drug license which is issued to a person who wants to sell drugs on a wholesale level.
A retail drug license is issued only to a person who possesses a degree or diploma in Pharmacy from a recognized university and registered with State Pharmacy Council.
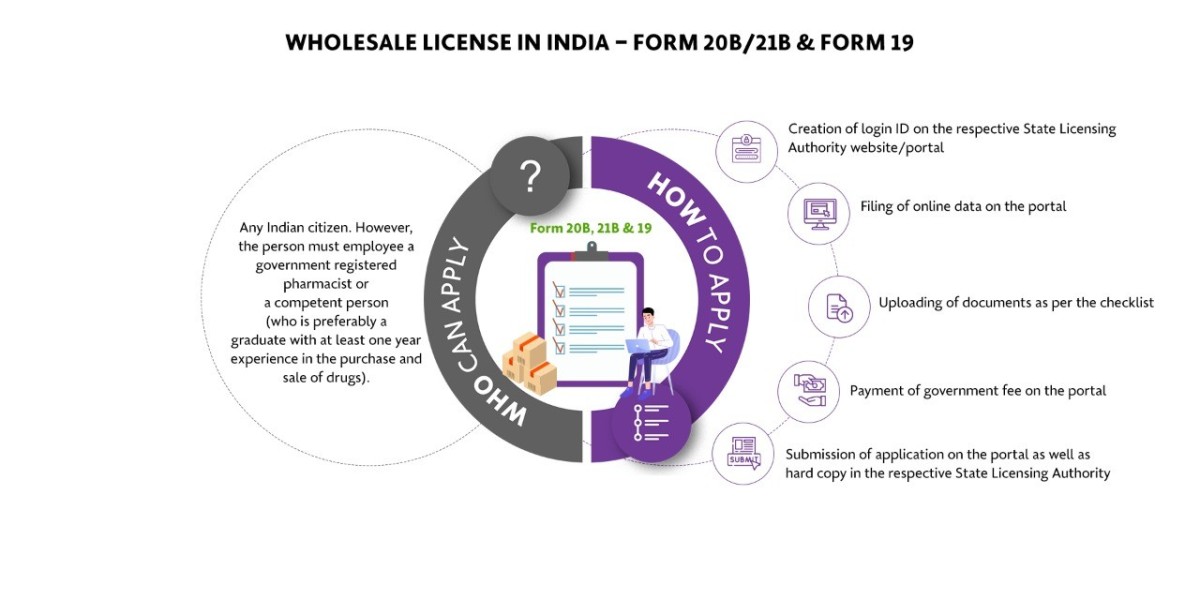
What is a Wholesale Drug License in India?
A wholesale Drug License is mandatory for pharmaceutical businesses that are doing business as wholesalers. Wholesalers are basically those who sell in bulk or in large quantities. They basically sell to retailers in large quantities for profit. For such kind of sale, they want permission from the concerned state authority in the form of a license. Hence we can say that permission taken by the wholesalers to do the pharmaceutical business in the form of a license is called a Wholesale Drug License.
What are the different types of drug licenses issued for the pharmaceutical business?
Based on the requirement of the pharmaceutical business, an applicant must apply for the issue of a specific drug license. There are various licenses, for example:
Wholesale Drug License,
Retail Drug License,
Import of Drugs or Cosmetics,
Import of Medical Equipment,
Export of Medicines etc.
Mandates To Get The Permission For Wholesale Drug License In India
- Obligatory for medicines, drugs, or cosmetics,
- Acknowledge commercial premises,
- Must comply with the requirements and terms imposed by authorities and law,
- It must be displayed all the time at the location of the business.
Basic Requirements To Obtain The Wholesale Drug License in India
For a company/distributor/independent agent to be an Authorized Indian Agent for a local/foreign manufacturer at the Central Drugs Standard Control Organization (CDSCO), they should hold a valid Wholesale Drug License in Form 20B and 21B. The wholesale drug license application is submitted to and approval granted by individual State Licensing Authorities. The Manufacturing License in India is essentially about the State Drug Licensing Authority, CDSCO Zonal/Sub-Zonal Office and Drugs Controller General of India CDSCO (HQ).
Some of the basic requirements for obtaining a wholesale drug license are,
- The required minimum space needed to open a wholesale pharmacy or Medical shop outlet for wholesale is 10 square meters.
- Certain medications, such as vaccines, insulin injections, etc., must be stored in the fridge. Therefore, it is required that you be a refrigerator as well as an air conditioner on the premises.
- A Wholesale Drug License can be made under the personal supervision of the registered pharmacist / competent person. In some states, a Wholesale Drug License is issued only to a company/entity with a person(s) degree or diploma in pharmacy from a recognized institute/university.
- The pharmacist or a competent person should be a graduate with 1-year experience in dealing drugs or be S.S.L.C passed with 4 years of experience in dealing drugs.
- Once the Drug License is obtained, the same should be displayed clearly on the premises for the purpose of any future inspection.
Important Document Requirement To Get Drug License
The required documents for the drug sale license are listed below. Application form no. 19 is to be submitted in a prescribed form.
- Covering letter signed with the name and designation of the applicant.
- Challan of fee deposited.
- Declaration form in the prescribed format.
- Key plans for the premises.
- Site plan for the premises.
- Affidavit of proprietor, partner, pharmacist etc.
- Refrigeratorpurchase bill with the address.
- Ownership or and rent agreement deed of premises.
- Electricity bill of the premises.
- Firm registration receipt in case of a private limited firm.
- Pharmacist/Competent person qualification document.
Document Requirement for New Wholesale Drug License
The required documents can differ based on each state. However, below is the list of generic document requirements for obtaining a wholesale drug license in India.
- System-generated application form for relevant drug sales (19/19A/19B/19C as applicable).
- Challan of Rs. 3000/- regarding the fee submission of the license.
- Affidavit of the proprietor on stamp paper of 20 Rs.
- Educational certificate copies of the proprietor (Self attested)
- Self-attested copies of Identity proof of proprietor (Domicile/ Driving license/ Voter ID card)
- Affidavit of Competent person.
- Educational certificate copies of Competent person (Self attested)
- Self-attested copies of Identity proof of a Competent Person (Domicile/ Driving license/ Voter ID card)
- Experience certificate of a competent person. (Experience certificate of 4 years after 12th or experience certificate of 2 years after graduation in original)
- Blueprint of a Plan layout of the proposed area/ premises.
- Electricity bill of proposed area/ premises.
- Copy of Refrigerator bill.
- Rent agreement (50 Rs. Stamp paper) for minimum 5 years
- Ownership document of the premises, including the copy of the tax receipt of the proposed area/ premises.
- Covering letter.
- Photo 5-5 each of the Competent person and proprietor if required.
Documents required for application of an additional wholesale license:
- Application form generated by the system for drug sales (19/19A/19B/19C in case applicable).
- Affidavit regarding non-conviction of Property/Partner/Director as well as the firm under Drugs & Cosmetics Act, 1940.
- Required Details for wholesale licenses:
- Documentation of qualifications, i.e. the final degree certificate or provisional certificate with marks sheets of a registered pharmacist.
- Registration at Pharmacy Council.
- Appointment Letter and Bio-data.
- System Generated Affidavit.
4. Online fee deposit receipts.
Documents needed for the application of renewal of Wholesale License:
- Application form generated by the system for drug sales (19/19A/19B/19C in the event of a need).
- The receipt for the conversion charge issued by the state government of state together with other pertinent documents supporting commercial use, as per MPD 2021, specifically copies displaying the name of the commercial/mixed-use road/street that has been notified.
- Online fee deposit receipts.
Note :
The applicant is required to establish an independent store with enough space for the grant of wholesale or retail licenses in accordance with the details listed below:
- In order to grant the license on Form 20 or Form 21 and both must be with a total area of at least 10 square meters.
- In order to grant authorization in form 20-B or form21-B, premises must be in an area no less than 10 square meters.
- Licenses are granted for the purpose of granting.
- On Forms 20 and Form 21 forms,
- In Form 20, Form 21, Form 20B, Form 21-B, or both forms, the property must have an area not less than 15 square meters.
The size of the premises for sale must be in line with the National Building Code of India 2005, which was updated from time to time.
For premises that are situated on a plot of land that is residential or flat, a drug license cannot be issued unless the property conforms to the standards.
All documents must be signed by the applicant or authorized person to confirm the authenticity of the document and that they have submitted identical documents.
The Step-by-Step Process to register Your Medical Device in India will establish if the product is required to be registered and must be a Notified Medical Device. If it is, proceed with the appointment of an approved Indian agent, submit the regulatory dossier, obtain the Registration Certificate, obtain an Import License and begin Marketing in India.