Further, we have also highlighted the Cooperative Patent Classification (CPC) symbols and leading players (in terms of number of patents filed / granted). Moreover, the module includes an informed patent benchmarking analysis of the top patent applicants, along with an analysis of the relative valuation of patents filed / granted related to viral clearance testing, based on several parameters, such as patent age, region in which patent is filed / granted and number of citations.
For the purpose of this analysis, information on patents was extracted from a reliable database, lens.org. The database groups patent applications into different patent families and covers intellectual property documents filed with different patent offices, across the globe. Around 65% of the patents filed related to viral clearance and viral testing were applications (not having received approval yet), followed by granted patents. It is worth highlighting that, the maximum number of patents (over 50) related to this domain were filed / granted in 2020. Further, close to 35 patents have been filed / granted in 2022 (till September)
To request a sample copy / brochure of this report, please visit this
Based on several relevant parameters, such as patent type, patent age, number of citations, and region, the patents were allotted different valuation scores. Our analysis indicates that only 1% of patents have the highest relative valuation, based on the parameters and the cut-off of the weighted score considered for this analysis. This is primarily because of the relatively low citations of the patents that have been filed recently, leading to low patent value.
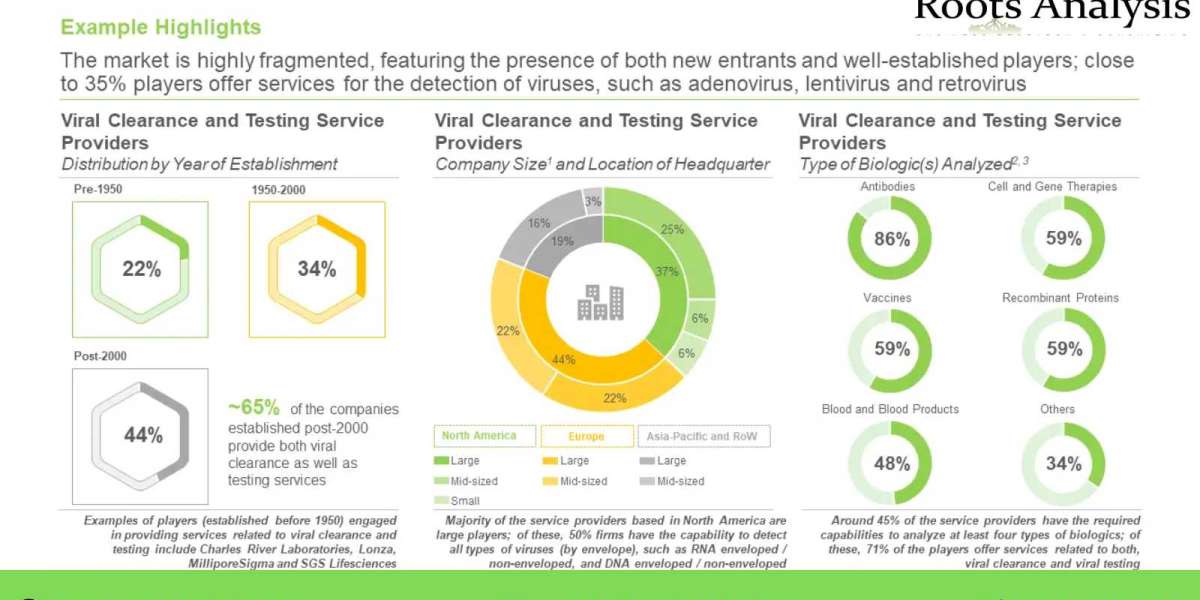
In terms of Geography, most of the patents (36%) were filed / granted in Asia-Pacific. Within this region, the maximum number of patents (over 40) were filed in China. This is followed by North America, where 32% of the total patents were filed / granted. The number of patents is indicative of the extensive research activity taking place in these regions. more than 85% of the players efficiently analyze antibodies for viral contamination, followed by players that have capability to analyze cell and gene therapies, vaccines and recombinant proteins (59%, each) for viral contamination
For additional details, please visit
https://www.rootsanalysis.com/reports/viral-clearance-and-testing-services-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com