Why are ISO 13485 Audit Services Essential for Medical Device Manufacturers?
For medical device manufacturers, achieving and maintaining ISO 13485 certification is vital for ensuring consistent quality and meeting regulatory requirements. ISO 13485:2016 sets the international standard for quality management systems specific to medical devices, ensuring that products are consistently designed, developed, produced, installed, and serviced in a way that meets customer and regulatory requirements.
Read More : https://sumssolution.com/why-a....re-iso-13485-audit-s
Discover posts
Mining Explosives Market Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032 | #mining Explosives Market
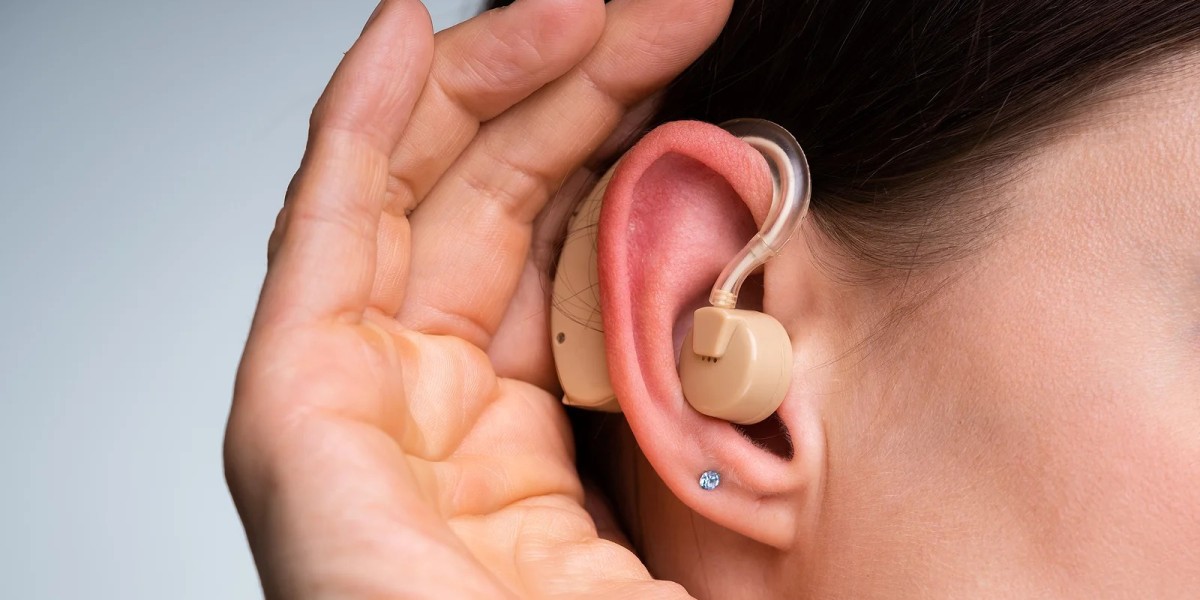
Audiological Devices Market: Key Hindrances Limiting Expansion and Adoption | #audiological devices market #audiological devices #hearing aids #hearing devices
Sustainable Fabrics Market Revenue, Business Segment Overview and Key Trends 2032 | #sustainable Fabrics Market
Juno Creative
After being in business for over 13 years, we know a thing or two about design. We live to create – outside of the 9-5, you’ll find us poring over design magazines and making way too many Pinterest boards. Our team is a versatile bunch, with skills ranging from branding and packaging to copywriting and photography (and everything in between).
Address: 1/30 Light St, Fortitude Valley, QLD 4006, Australia
Phone: +61 7 3257 1115
Website: https://junocreative.net.au

Color Stainless Wall Cladding Casinos | #color Stainless Wall Cladding
Chrome Plating Market Opportunity, Demand, Growth, Analysis and Forecast by 2032 | #chrome Plating Market
Taurus and Libra Compatibility: A Vibrant Match Made in Zodiac Heaven!
"Taurus and Libra" investigates the unique association between two zodiac signs administered by Venus, the planet of adoration, magnificence, and agreement. Taurus, an earth sign, values soundness, arousing quality, and reasonableness, while Libra, an air sign, blossoms with balance, basic show of manners, and scholarly associations. Together, they make an interesting bond where Taurus gives establishing and dedication, and Libra brings appeal and strategy. This matching features their true capacity for profound close to home understanding and shared adoration, while likewise digging into the difficulties they face in mixing their particular ways to deal with life. An excursion of affection, balance, and shared development unfurls in this heavenly association.
visit us:https://www.sagaciajewelry.com..../blogs/news/taurus-a

How to 're register DSC on Income Tax portal
#dscregistrationintrichy
To re-register DSC on the Income Tax portal, log in, go to the Profile Settings, select Digital Signature, and upload your DSC file. To know further, contact Shoplegal, +91 9751110101.
http://www.shoplegal.in/digita....l-signature-certific

Edit Offer
Add tier
Delete your tier
Reviews
Pay By Wallet
Payment Alert
You are about to purchase the items, do you want to proceed?