Plasma Protease C1-inhibitor Treatment Market Overview
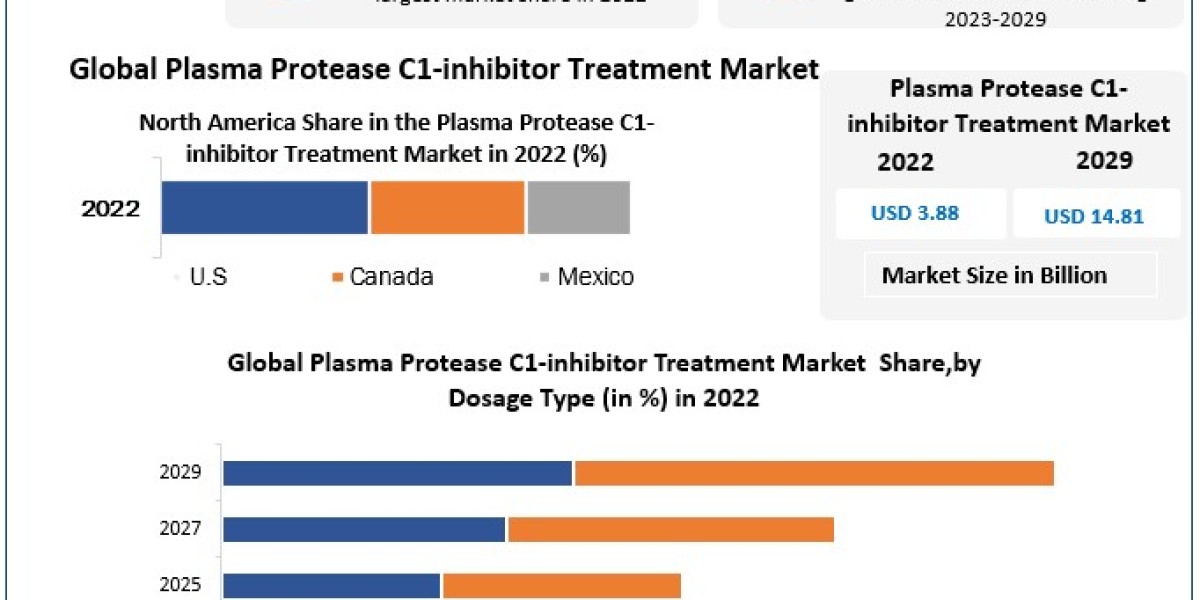
The global Plasma Protease C1-inhibitor Treatment Market Trends is poised for significant growth, with projections indicating a rise from USD 3.88 billion in 2022 to approximately USD 14.81 billion by 2029, reflecting a robust compound annual growth rate (CAGR) of 18.2% during the forecast period. This expansion is primarily driven by the increasing prevalence of hereditary angioedema (HAE), advancements in therapeutic options, and heightened awareness of rare genetic disorders.
Grab your sample copy of this report now: https://www.maximizemarketresearch.com/request-sample/36386/
Market Overview & Definition
Plasma protease C1-inhibitor (C1-INH) treatment, also known as C1-INH replacement therapy, is a medical intervention used to manage conditions associated with C1-INH deficiency, notably hereditary angioedema (HAE). HAE is a rare genetic disorder characterized by recurrent episodes of severe swelling in various body parts, including the extremities, face, gastrointestinal tract, and airways. This condition arises from a deficiency or dysfunction of the C1-INH protein, leading to excessive production of bradykinin, a peptide responsible for increasing vascular permeability and subsequent edema. C1-INH therapies work by inhibiting enzymes involved in the complement, coagulation, and kallikrein-kinin systems, thereby reducing bradykinin production and preventing angioedema attacks. These treatments are available in both intravenous and subcutaneous formulations, offering flexibility in administration to meet patient needs.
Market Growth Drivers & Opportunities
Several key factors are propelling the growth of the plasma protease C1-inhibitor treatment market:
Increasing Prevalence of Hereditary Angioedema (HAE): The global incidence of HAE is rising, necessitating effective therapeutic interventions. Improved diagnostic capabilities and greater awareness among healthcare providers have led to earlier identification and treatment of HAE, thereby increasing the demand for C1-INH therapies.
Advancements in Therapeutic Options: Continuous research and development efforts have resulted in the introduction of novel C1-INH formulations and administration routes. For instance, the approval of subcutaneous C1-INH products offers patients more convenient and less invasive treatment options, enhancing compliance and quality of life.
Growing Investment in Drug Development: Pharmaceutical companies are investing heavily in the development of innovative C1-INH therapies. Promising pipeline drugs and novel therapeutic approaches are expected to expand the treatment landscape, providing patients with more effective and tailored options.
Increased Awareness and Education: Initiatives aimed at educating healthcare professionals and the public about HAE have led to improved recognition and management of the condition. This heightened awareness facilitates timely treatment interventions, thereby driving market growth.
Request a Free Sample Copy or View Report Summary: https://www.maximizemarketresearch.com/request-sample/36386/
Plasma Protease C1-inhibitor Treatment Market Segmentation
The plasma protease C1-inhibitor treatment market is segmented based on drug class, dosage type, and distribution channel:
By Drug Class:
- C1-Inhibitors: This segment holds the dominant market share, attributed to the widespread use of C1-INH products in both prophylactic and acute settings for HAE management.
- Kallikrein Inhibitors (e.g., Kalbitor): These agents are gaining traction due to their role in inhibiting kallikrein, an enzyme involved in bradykinin production, thus preventing angioedema attacks.
- Selective Bradykinin B2 Receptor Antagonists (e.g., Firazyr): These drugs block the bradykinin B2 receptor, mitigating the effects of bradykinin and providing relief from acute HAE symptoms.
By Dosage Type:
- Lyophilized Formulations: These products are reconstituted prior to administration and are commonly used in intravenous therapies.
- Liquid/Injectable Formulations: Ready-to-use injectable forms, including subcutaneous options, offer convenience and are associated with increased patient adherence.
By Distribution Channel:
- Hospital Pharmacies: Hospitals remain a primary distribution channel, especially for acute care and inpatient settings.
- Independent Pharmacies and Outlets: Retail pharmacies provide accessibility for outpatient and home-based therapies, catering to the growing trend of self-administration.
Country-Level Analysis
United States: North America, particularly the U.S., dominates the plasma protease C1-inhibitor treatment market, driven by a high prevalence of HAE, advanced healthcare infrastructure, and significant investment in research and development. The region's focus on early diagnosis and proactive management of HAE contributes to its leading market position.
Germany: As a key player in the European market, Germany's growth is propelled by robust healthcare systems, extensive research initiatives, and a strong emphasis on rare disease management. Collaborative efforts between academic institutions and pharmaceutical companies foster innovation and accessibility to advanced C1-INH therapies.
For More Information About This Research Please Visit: https://www.maximizemarketresearch.com/request-sample/36386/
Competitive Analysis
The plasma protease C1-inhibitor treatment market is characterized by the presence of several key players focusing on innovation, strategic partnerships, and expansion to strengthen their market positions. Notable companies include:
Shire plc (part of Takeda Pharmaceutical Company Limited): A leader in the market, Shire offers a comprehensive portfolio of C1-INH products, including both intravenous and subcutaneous formulations. Their commitment to research and patient-centric solutions has solidified their market presence.
CSL Limited: Known for their product Berinert, CSL provides C1-INH therapies approved for acute HAE attacks. Ongoing investments in research and development underscore their dedication to advancing treatment options.
Pharming Group N.V.: The company markets Ruconest, a recombinant C1-INH therapy. Pharming's focus on biotechnological innovations positions them as a significant contributor to the market.
Sanquin: As a not-for-profit organization, Sanquin is involved in the production of plasma-derived C1-INH products, contributing to the availability of essential therapies for HAE patients.
Latest cutting-edge research from Maximize Market Research is now trending:
Global Medical Lighting Technologies Market https://www.maximizemarketresearch.com/market-report/global-medical-lighting-technologies-market/22069/
Global Biomedical Warming and Thawing Devices Market https://www.maximizemarketresearch.com/market-report/global-biomedical-warming-and-thawing-devices-market/101076/
Latin America Blood Glucose Monitoring System Market https://www.maximizemarketresearch.com/market-report/blood-glucose-monitoring-system-market-latin-america/1697/
Contact Maximize Market Research:
3rd Floor, Navale IT Park, Phase 2
Pune Bangalore Highway, Narhe,
Pune, Maharashtra 411041, India
Email: sales@maximizemarketresearch.com
+91 96071 95908, +91 9607365656