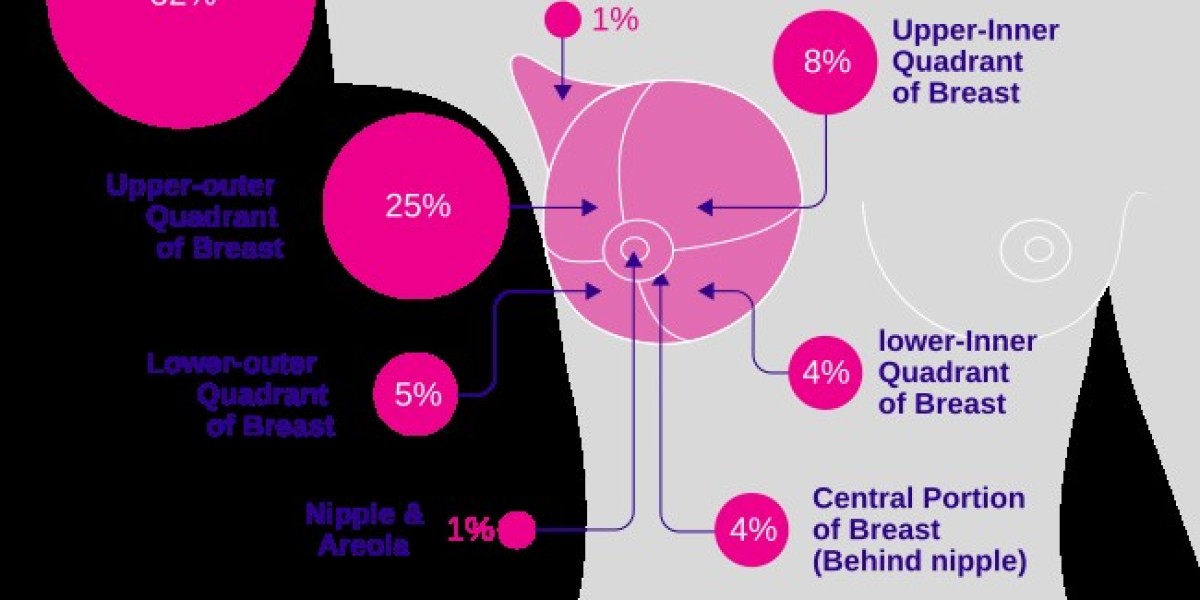
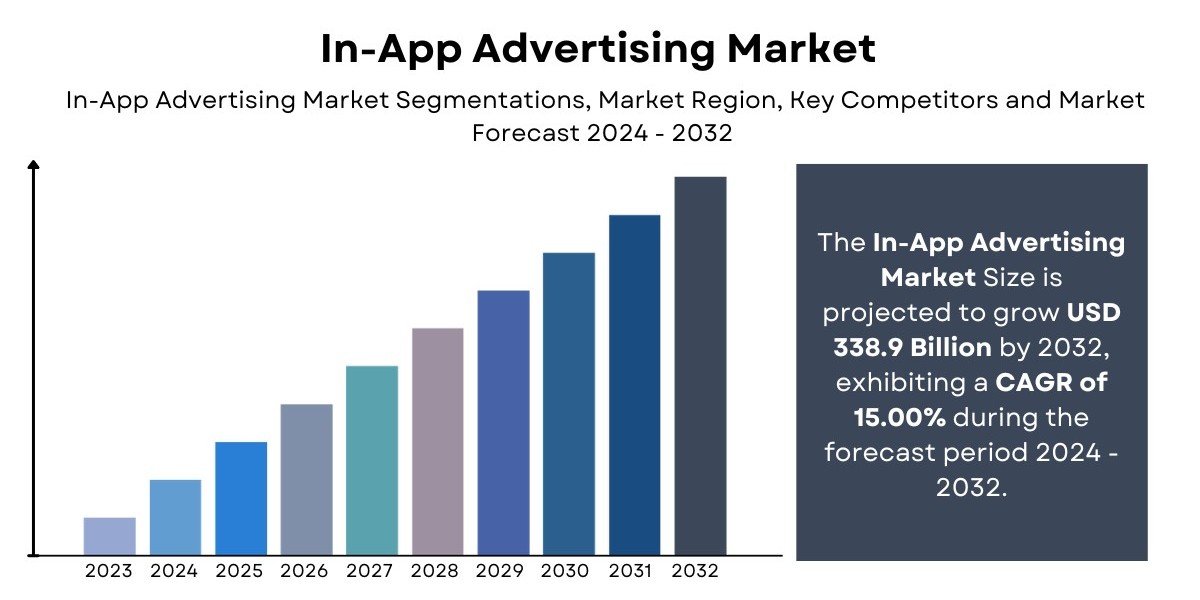
Introduction
Breast cancer remains one of the most formidable health challenges worldwide, with the ER-positive, HER2-negative (ER+ HER2-) subtype posing unique treatment hurdles. In recent years, the advent of precision medicine has revolutionized the way clinicians approach this disease, shifting from broad-spectrum therapies toward targeted interventions that align with a tumor’s unique genetic profile. At the forefront of this transformation is Lynparza, a breakthrough oral PARP inhibitor whose active ingredient, olaparib, has redefined treatment paradigms for patients battling advanced breast cancer. This article explores the clinical impact of Lynparza, detailing its mechanism of action, clinical efficacy and safety, market performance, and future innovations that continue to reshape breast cancer outcomes.
For more in-depth insights on Lynparza’s development and future potential, download the full report @ Lynparza Market Report.
What is Lynparza?
Lynparza is an innovative oral therapy developed by AstraZeneca and Merck, designed specifically to target cancer cells with compromised DNA repair mechanisms. With olaparib as its active ingredient, Lynparza was initially approved for the treatment of ER+ HER2- metastatic breast cancer in patients with germline BRCA mutations (gBRCAm) who have already undergone chemotherapy or endocrine therapy. Its role, however, has expanded beyond metastatic settings; Lynparza is now also indicated as adjuvant therapy for high-risk early-stage breast cancer. This dual application underscores its versatility and the commitment of precision oncology to tailor treatment based on individual genetic profiles.
The approval process for Lynparza was underpinned by rigorous biomarker-driven patient selection, utilizing companion diagnostic tests that identify patients with homologous recombination deficiency (HRD) or BRCA mutations. Such an approach not only maximizes the therapeutic benefits for those most likely to respond but also minimizes unnecessary exposure to potential side effects. In this way, Lynparza exemplifies how precision medicine is being harnessed to improve outcomes in breast cancer care.
Lynparza Mechanism of Action (MOA)
Central to Lynparza’s success is its unique mechanism of action—a process grounded in the principle of synthetic lethality. Under normal conditions, PARP enzymes are critical for repairing single-strand breaks in DNA. However, in cancer cells harboring BRCA mutations or exhibiting HRD, the inhibition of these enzymes by Lynparza’s active ingredient, olaparib, leads to an accumulation of DNA damage. Without the ability to effectively repair these breaks, the cancer cells incur lethal double-strand breaks that ultimately result in cell death.
This targeted disruption of DNA repair processes is particularly effective in ER+ HER2- breast cancers, where genetic vulnerabilities are common. By specifically exploiting these vulnerabilities, Lynparza offers a therapeutic advantage over traditional chemotherapies, which often affect both cancerous and healthy cells. Moreover, preclinical studies have demonstrated the potential for synergy between Lynparza and platinum-based therapies, suggesting that combining these treatments may further enhance clinical outcomes.
Lynparza’s Mechanism of Action not only illustrates the power of precision oncology but also highlights the importance of its active ingredient, olaparib, in transforming the management of breast cancer. As oncologists increasingly integrate biomarker-driven strategies into clinical practice, the significance of such targeted approaches continues to grow.
For more detailed insights and the latest updates on Lynparza, visit the Lynparza Market update.
Clinical Efficacy and Safety
The clinical efficacy of Lynparza has been firmly established through a series of robust clinical trials—often referenced as Lynparza Clinical Trials—which have consistently shown improvements in progression-free survival and overall disease management. In metastatic settings, Lynparza has proven to be a highly effective maintenance therapy following chemotherapy, delaying disease progression and providing patients with a better quality of life. Its use in the adjuvant setting further demonstrates its capacity to significantly reduce the risk of cancer recurrence in high-risk patients.
Equally important is the safety profile of Lynparza. Although patients may experience side effects such as fatigue or anemia, these adverse events are generally manageable with appropriate dose adjustments and careful monitoring. The favorable safety profile is a testament to the drug’s design—targeting only cancer cells with defective DNA repair while largely sparing normal, healthy cells. As HRD testing becomes a routine part of the diagnostic process, clinicians can better select patients who are most likely to benefit from Lynparza, thereby optimizing treatment outcomes while minimizing toxicity.
Lynparza Cost and Accessibility
As with many advanced therapies, Lynparza is associated with a higher upfront cost reflective of its innovative status. However, its long-term benefits—such as delayed disease progression, reduced hospitalization rates, and improved survival outcomes—offer a compelling economic value proposition. Health insurers have increasingly recognized this value, leading to expanded coverage particularly for patients with confirmed gBRCAm status. In addition, manufacturer assistance programs have been implemented to help mitigate out-of-pocket expenses, ensuring that more patients have access to this transformative therapy.
The ongoing push for universal HRD testing is another critical factor in enhancing accessibility. By broadening the identification of eligible patients, healthcare systems can ensure that Lynparza reaches those who stand to benefit most. This approach not only improves clinical outcomes but also supports a more efficient allocation of healthcare resources in the battle against breast cancer.
For further insights and detailed research on this breakthrough treatment, visit Lynparza Insights.
Lynparza Sales and Market Performance
In the competitive arena of oncology therapeutics, Lynparza has carved out a dominant position, with its success reflected in impressive Lynparza sales figures. These sales are driven by several key factors: robust clinical efficacy, strategic regulatory approvals, and targeted educational campaigns that have raised awareness among oncologists. Notably, the strategic partnership between AstraZeneca and Merck has been instrumental in expanding the global reach of Lynparza, ensuring that it is accessible to patients in diverse markets around the world.
Recent Lynparza Approvals have further solidified its status, expanding its indications and reinforcing its market leadership in both metastatic and adjuvant settings. The impressive trajectory of Lynparza sales is expected to continue as more clinicians embrace biomarker-driven therapies and as ongoing clinical research broadens its therapeutic applications. With strong market performance and sustained demand, Lynparza sales have become a critical barometer of the ongoing shift toward precision medicine in breast cancer treatment.
The strategic emphasis on Lynparza sales in market analyses reflects the drug’s dual role as both a clinical breakthrough and a commercial success. As the landscape of oncology evolves, Lynparza sales serve as a powerful indicator of the broader trend toward personalized, targeted therapies in modern cancer care.
Future Outlook and Innovations
The future of Lynparza is marked by continued innovation and expansion into new therapeutic frontiers. Ongoing clinical trials are investigating novel combination therapies that pair Lynparza with immunotherapy agents and CDK4/6 inhibitors, aiming to overcome resistance mechanisms and further improve patient outcomes. These efforts are complemented by advances in diagnostic technologies, such as liquid biopsy, which promise to refine HRD detection and broaden the scope of patient eligibility.
Beyond breast cancer, research is actively exploring the use of Lynparza in other HRD-positive malignancies, including gastric and bladder cancers. Such investigations are expected to lead to additional Lynparza Approvals, expanding the therapeutic reach of this precision medicine. The integration of new biomarkers and the refinement of patient selection criteria will continue to drive the evolution of Lynparza Clinical Trials, ensuring that its benefits extend to a wider range of patients.
As the oncology community moves toward a more personalized approach to cancer treatment, Lynparza stands as a beacon of innovation. Its continued development, bolstered by a strong foundation of clinical data and market performance, positions it at the forefront of a new era in cancer care. The promise of combination therapies and next-generation diagnostic tools heralds a future in which Lynparza not only reshapes breast cancer outcomes but also serves as a model for the successful application of precision oncology across a spectrum of malignancies.
For additional insights on Lynparza’s transformative potential, please download the full Lynparza report.
Conclusion
Lynparza has undeniably reshaped the landscape of breast cancer treatment, emerging as a transformative agent in the era of precision oncology. Its pioneering use of targeted DNA repair inhibition—anchored by its active ingredient, olaparib—has set a new standard for the management of ER+ HER2- breast cancer. From its well-defined Mechanism of Action to its robust clinical efficacy demonstrated in Lynparza Clinical Trials, this therapy exemplifies the promise of biomarker-driven treatment.
The favorable safety profile, combined with strategic Lynparza sales initiatives and expanding regulatory approvals, underscores its impact on both clinical practice and the broader oncology market. As the cost and accessibility issues are addressed through expanded insurance coverage and manufacturer assistance programs, more patients are poised to benefit from this innovative therapy.
Looking ahead, ongoing clinical research and technological advancements are set to further enhance Lynparza’s therapeutic potential. With new combination regimens and broader diagnostic capabilities on the horizon, Lynparza is well-positioned to continue reshaping breast cancer outcomes and extend its benefits to other HRD-positive cancers. Ultimately, the clinical impact of Lynparza is measured not only in improved survival rates and quality of life but also in its role as a catalyst for a more personalized, targeted approach to cancer care.
For those looking to explore this breakthrough treatment more, download the full Lynparza Insights Report.
Read More
- Lynparza - API Insight
- Metastatic HR+/HER2− Breast Cancer - Market Insights, Epidemiology, and Market Forecast
- Metastatic HER2 positive Breast Cancer - Market Insight, Epidemiology And Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.