Clinical Trial Management System (CTMS) Market, by Component (Software, Services), Delivery Mode (Cloud-based (SaaS) CTMS, Licensed Enterprise (On-premise) CTMS, Web-based (Hosted) CTMS), Type (Enterprise, Site), End User (Clinical Research Organizations (CROs), Medical Device Firms, Pharmaceutical & Biotechnology Firms, Other) and Region (North America, Europe, Asia-Pacific, and the Rest of the World).
"Streamlining Trials: Navigating the Clinical Trial Management System Market"
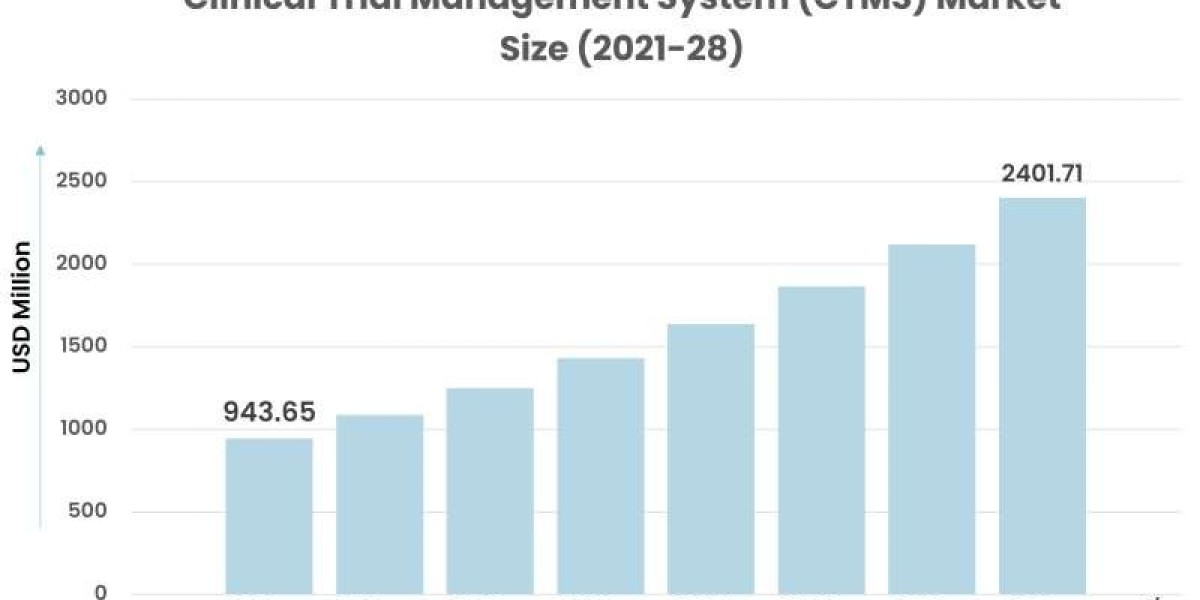
In the realm of medical research, efficiency and accuracy are paramount. The Clinical Trial Management System (CTMS) Market serves as a pivotal tool in achieving these objectives, streamlining the complex processes involved in conducting clinical trials. CTMS software offers a comprehensive suite of features designed to facilitate planning, monitoring, and reporting of clinical trials, thereby enhancing operational efficiency and ensuring regulatory compliance.
Navigating the CTMS Market involves understanding the diverse needs of research organizations and selecting the right solution to meet those requirements. These systems offer functionalities such as patient recruitment tracking, protocol management, adverse event reporting, and document management, all of which are crucial for the successful execution of clinical trials.
Moreover, the CTMS Market is witnessing significant advancements, driven by the integration of artificial intelligence, machine learning, and data analytics capabilities. These innovations enable predictive analytics, real-time monitoring of trial progress, and identification of potential risks, empowering researchers to make informed decisions and adapt protocols as needed.
By streamlining trial management processes, CTMS solutions contribute to shorter trial durations, reduced costs, and improved data quality. Additionally, they enhance collaboration among stakeholders, including sponsors, investigators, and regulatory authorities, fostering a more efficient and transparent research ecosystem.
In conclusion, navigating the CTMS Market involves embracing innovative solutions that streamline trial management processes, ultimately accelerating the pace of medical research and bringing life-saving treatments to patients more swiftly and efficiently.