With a compound annual growth rate (CAGR) of 4%, the global market for Hernia Mesh Devices is expected to reach US$ 6.99 billion by 2033 from its estimated valuation of US$ 4.72 billion in 2023. Due to the fact that hernia cases are rising dramatically worldwide, major players in the hernia mesh repair industry are vying to create more accessible hernia repair tools.
The global hernia mesh devices market continues to witness significant growth, driven by evolving market trends, technological advancements, and a growing patient population. With a surge in demand for minimally invasive procedures and a rise in hernia prevalence worldwide, the market is poised for steady expansion in the coming years.
Get Free Sample Research Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=8345
Key Companies Profiled
- Aspide Medical
- Atrium
- Braun Melsungen AG.
- Baxter International, Inc.
- R. Bard, Inc.
- Cook Medical
- Cousin Biotech
- Covidien (Part of Medtronic)
- Dipromed
- Ethicon, Inc.
- Feg Textiltechnik MBH
- Herniamesh S.r.l
- LifeCell Corporation
- L. Gore & Associates
Market Trend:
One of the prominent trends shaping the hernia mesh devices market is the increasing adoption of advanced surgical techniques, including laparoscopic and robotic-assisted procedures. These techniques offer several advantages such as reduced post-operative pain, shorter hospital stays, and faster recovery times, driving their widespread acceptance among both patients and healthcare providers.
Moreover, there's a growing preference for biologic meshes over synthetic counterparts due to their superior biocompatibility and reduced risk of complications such as infection and mesh rejection. This shift towards biologic meshes is expected to fuel market growth, especially in developed regions where healthcare infrastructure and reimbursement policies support the adoption of innovative medical technologies.
Hernia Mesh Devices Industry Research Segmentation:
- By Hernia Type :
- Inguinal
- Incisional
- Femoral
- By Mesh Type :
- Synthetic
- Biological
- By Procedure :
- Open Surgeries
- Laparoscopic Surgeries
- Robotic Surgeries
- By End-user :
- Hospitals
- Ambulatory Surgical Centers
- Clinics
- By Region :
- North America
- Latin America
- Europe
- East Asia
- South Asia & Oceania
- MEA
Market Analysis:
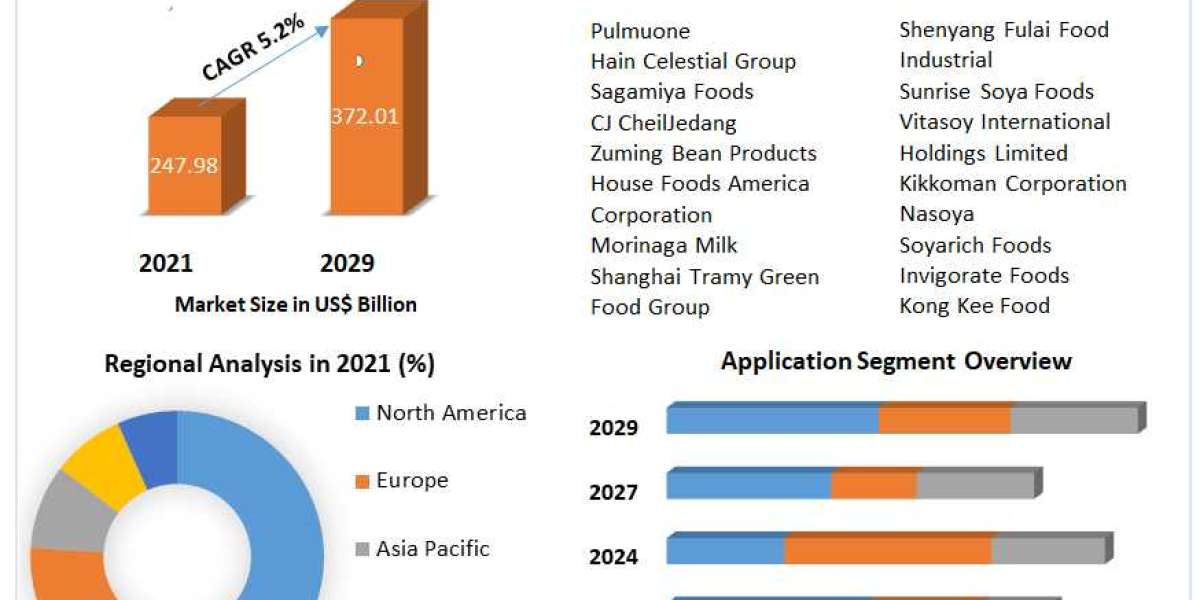
According to recent market studies, the global hernia mesh devices market is projected to register a compound annual growth rate (CAGR) of over 5% during the forecast period. Factors such as the increasing incidence of hernias, rising geriatric population, and advancements in mesh materials and design are contributing to market expansion.
North America currently leads the global hernia mesh devices market, attributed to the high prevalence of hernias, well-established healthcare infrastructure, and favorable reimbursement policies. However, emerging economies in Asia-Pacific and Latin America are expected to witness robust growth, driven by improving healthcare access, rising disposable incomes, and growing awareness about hernia treatment options.
Notable Developments:
Recent developments in the hernia mesh devices market include the introduction of innovative mesh materials with enhanced biomechanical properties and reduced inflammatory responses. Manufacturers are focusing on product innovation to address the unmet needs of patients and surgeons, thereby gaining a competitive edge in the market.
Furthermore, strategic collaborations and partnerships between medical device companies and healthcare institutions are facilitating the development of novel hernia repair solutions. These collaborations aim to leverage technological expertise and clinical insights to improve patient outcomes and streamline surgical procedures.
Opportunities, Challenges, and Concerns:
While the hernia mesh devices market presents lucrative opportunities for stakeholders, several challenges and concerns persist. Regulatory scrutiny surrounding the safety and efficacy of mesh implants, particularly in light of reported complications such as chronic pain and mesh migration, remains a key concern for manufacturers and healthcare providers.
Additionally, disparities in healthcare access and reimbursement policies across regions pose challenges to market penetration, particularly in developing countries where access to advanced medical treatments may be limited. However, efforts to raise awareness about hernia prevention and treatment options present opportunities for market growth, especially in underserved communities.
Get Free Sample Research Report: https://www.factmr.com/report/hernia-mesh-devices-market
Sustainable Solutions:
Addressing concerns related to the environmental impact of hernia mesh devices is gaining traction within the industry. Manufacturers are exploring eco-friendly materials and sustainable manufacturing processes to minimize the carbon footprint of mesh implants. Moreover, initiatives aimed at recycling and proper disposal of medical waste are being implemented to reduce environmental pollution and promote sustainability in healthcare practices.
Regional Trends:
Regional variations in healthcare infrastructure, regulatory frameworks, and patient demographics influence the dynamics of the hernia mesh devices market. While developed regions like North America and Europe continue to dominate in terms of market share, rapid urbanization and improving healthcare access in Asia-Pacific and Latin America are driving market expansion in these regions.
Related Publish by Fact.MR Industry:
Medical Processing Seals Market
https://www.factmr.com/report/medical-processing-seals-market
Atherectomy Device Market
https://www.factmr.com/report/atherectomy-device-market
Malignant Mesothelioma Market
https://www.factmr.com/report/malignant-mesothelioma-market
Oral Solid Dosage Contract Manufacturing Market
https://www.factmr.com/report/oral-solid-dosage-contract-manufacturing-market
????? ????.??
We are a trusted research partner of 80% of fortune 1000 companies across the globe. We are consistently growing in the field of market research with more than 1000 reports published every year. The dedicated team of 400-plus analysts and consultants is committed to achieving the utmost level of our client's satisfaction.
???????:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com