Introduction
In recent years, the field of gene therapy has witnessed remarkable advancements, with viral vectors emerging as pivotal tools for delivering therapeutic genes into target cells. Viral vectors, derived from viruses such as adeno-associated virus (AAV), lentivirus, and adenovirus, offer several advantages including efficient gene delivery and long-term gene expression. However, their successful application in clinical settings hinges upon effective purification strategies.
Definition
Removing contaminants and condensing viral vectors for use in gene therapy or vaccine development is known as viral vector purification. Typically, methods like chromatography, filtration, and ultracentrifugation are used to separate and refine the virus particles in order to guarantee their effectiveness and safety in biomedical settings.
Challenges in Viral Vector Purification
- Host Cell Contaminants: During viral vector production, host cell proteins and DNA can co-purify with the vector particles, potentially compromising their safety and efficacy. Removing these contaminants while preserving the integrity of the viral vectors poses a significant challenge.
- Viral Vector Heterogeneity: Viral vector populations often exhibit heterogeneity in terms of size, shape, and surface characteristics. This variability complicates purification processes, as conventional purification techniques may not adequately separate different vector species.
- Scalability: Many purification methods developed for research-scale production may not be scalable for large-scale manufacturing. Achieving high yields of purified viral vectors in a cost-effective and time-efficient manner remains a critical challenge for industrial-scale production.
- Purity and Safety: Ensuring the purity and safety of viral vectors is paramount for their clinical application. Residual host cell DNA, proteins, endotoxins, and other impurities must be meticulously removed to meet regulatory standards and minimize the risk of adverse immune responses in patients.
Solutions to Overcome Challenges
- Column Chromatography: Size-exclusion chromatography (SEC), ion exchange chromatography (IEX), and affinity chromatography are commonly employed for viral vector purification. These techniques exploit differences in size, charge, or affinity to separate viral vectors from contaminants. Advances in column design and media chemistry have improved the resolution and efficiency of chromatographic purification methods.
- Ultrafiltration and Diafiltration: Tangential flow filtration (TFF) using ultrafiltration membranes enables the concentration and buffer exchange of viral vector suspensions. Diafiltration further enhances purity by continuously diluting and replacing impurities with fresh buffer solution. TFF systems can be scaled up for large-scale manufacturing, offering versatility and efficiency in viral vector purification.
- Density Gradient Ultracentrifugation: Ultracentrifugation through density gradients allows for the separation of viral vectors based on their buoyant density. This technique can effectively remove host cell contaminants and isolate viral vectors with high purity. However, its scalability and throughput may be limited in industrial settings.
- Precipitation Methods: Salting-out precipitation and polyethylene glycol (PEG) precipitation exploit differences in solubility to selectively precipitate viral vectors from solution. While these methods can yield high-purity viral vector preparations, they often require extensive optimization and may not be suitable for large-scale production due to their batch-wise nature.
- Combination Strategies: Integrated purification platforms combining multiple techniques offer synergistic advantages in terms of purity, yield, and scalability. For example, a sequential approach involving chromatography followed by ultrafiltration/diafiltration can effectively remove impurities while concentrating viral vectors to desired levels.
Future Directions
As the demand for viral vectors in gene therapy continues to grow, ongoing research efforts are focused on developing innovative purification technologies that address current challenges and streamline manufacturing processes. Emerging trends such as continuous purification systems, novel affinity ligands, and process analytical technologies hold promise for enhancing the efficiency and robustness of viral vector purification.
Growth Rate of Viral Vector Purification Market
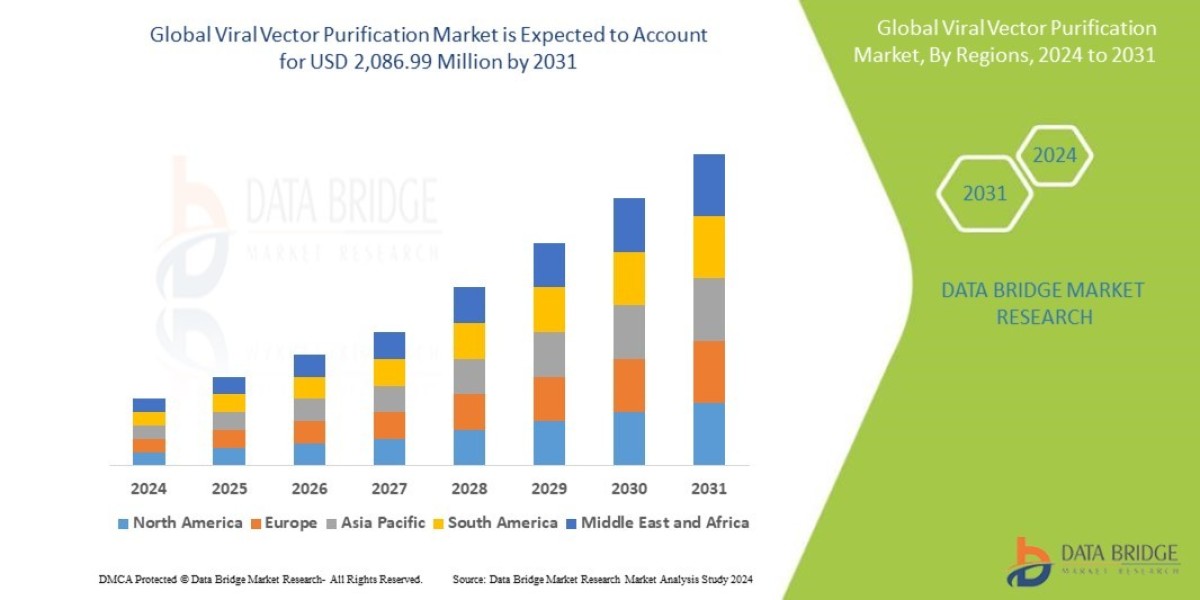
According to Data Bridge Market Research's analysis, the global market for viral vector purification, which was valued at USD 721.42 million in 2023, is anticipated to grow at a compound annual growth rate (CAGR) of 14.2% from 2024 to 2031, reaching USD 2,086.99 million.
Conclusion
Viral vector purification remains a critical step in the development and manufacturing of gene therapy products. Addressing key challenges such as host cell contaminants, heterogeneity, scalability, and safety requires a multifaceted approach integrating advanced purification techniques and innovative process design. By overcoming these hurdles, researchers and manufacturers can accelerate the translation of viral vector-based therapies from the laboratory to the clinic, ultimately improving patient outcomes and advancing the field of gene therapy as a whole.
To read more click here.
https://www.databridgemarketresearch.com/reports/global-viral-vector-purification-market