The European In Vitro Diagnostics market is experiencing significant growth, propelled by a wave of technological advancements. These innovations are enhancing the accuracy, speed, and accessibility of diagnostic tests, ultimately improving patient care and driving market expansion. This article explores the key technological advancements that are boosting the European IVD market.
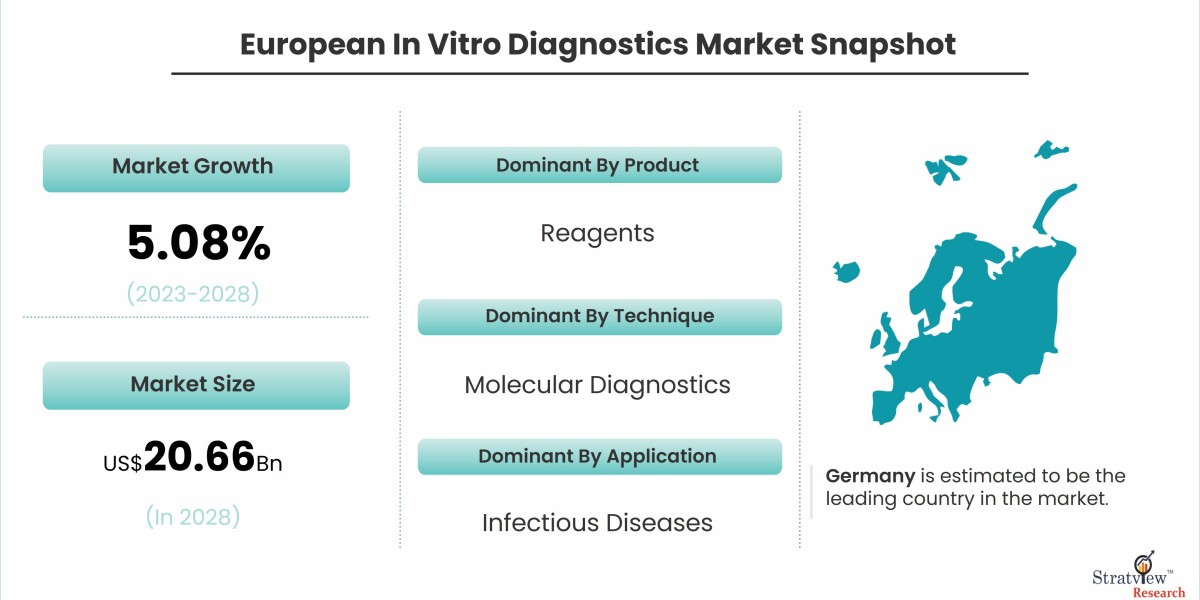
According to Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
Next-Generation Sequencing (NGS)
Next-generation sequencing (NGS) has revolutionized the field of genomics, enabling comprehensive analysis of genetic material with high accuracy and speed. NGS technology allows for the detection of genetic mutations, identification of pathogens, and understanding of complex diseases at a molecular level. Its applications in oncology, rare genetic disorders, and infectious diseases are particularly impactful. The ability to perform large-scale genomic sequencing at a lower cost is driving the adoption of NGS in clinical settings across Europe, contributing to the growth of the IVD market.
Digital Pathology
Digital pathology is transforming traditional pathology by converting glass slides into digital images that can be viewed, analyzed, and shared electronically. This technology improves diagnostic accuracy and efficiency, enabling pathologists to collaborate remotely and access specialized expertise regardless of location. Digital pathology also facilitates the use of artificial intelligence (AI) algorithms for image analysis, enhancing diagnostic precision. The adoption of digital pathology in Europe is increasing, supported by advancements in imaging technology and AI, which are driving market growth.
Point-of-Care Testing (POCT)
Point-of-care testing (POCT) is gaining prominence due to its ability to provide rapid diagnostic results outside traditional laboratory settings. POCT devices are becoming more sophisticated, offering a wide range of tests that can be conducted in clinics, emergency rooms, and even at home. These tests deliver immediate results, enabling timely clinical decision-making and improving patient outcomes. Technological advancements in miniaturization, biosensors, and connectivity are driving the expansion of POCT in Europe, making it a crucial growth driver for the IVD market.
Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning are revolutionizing the IVD sector by enhancing diagnostic accuracy and operational efficiency. AI algorithms can analyze vast amounts of data quickly and accurately, identifying patterns and predicting disease outcomes. In areas such as radiology, pathology, and genomics, AI-driven tools are proving to be invaluable. The integration of AI in diagnostic processes is expected to continue accelerating, providing more robust and reliable diagnostic solutions and boosting the European IVD market.
Liquid Biopsy
Liquid biopsy is an emerging technology that allows for the detection of cancer and other diseases through a simple blood test. Unlike traditional biopsies, which require invasive tissue sampling, liquid biopsies analyze circulating tumor DNA (ctDNA) or other biomarkers present in the blood. This non-invasive approach enables early detection, monitoring of treatment responses, and detection of disease recurrence. Technological advancements in molecular biology and genomics are making liquid biopsies more accurate and accessible, driving their adoption in Europe and contributing to the growth of the IVD market.
Automation and Robotics
Automation and robotics are playing a crucial role in improving the efficiency and accuracy of laboratory diagnostics. Automated systems can handle high volumes of samples with minimal human intervention, reducing the risk of errors and increasing throughput. Robotics are being used for tasks such as sample preparation, pipetting, and data analysis. The integration of automation and robotics in IVD laboratories is streamlining workflows, reducing costs, and enhancing diagnostic capabilities, driving market growth in Europe.
Telemedicine and Remote Diagnostics
The COVID-19 pandemic has accelerated the adoption of telemedicine and remote diagnostics, and this trend is expected to continue. Remote diagnostic tools, facilitated by digital health platforms, allow patients to receive medical advice and testing without visiting healthcare facilities. Advances in telecommunication and mobile health technologies are making remote diagnostics more accessible and efficient. This shift not only improves patient convenience but also expands access to healthcare services, particularly in remote and underserved areas, boosting the IVD market.
Conclusion
Technological advancements are significantly boosting the European In Vitro Diagnostics market by enhancing diagnostic accuracy, speed, and accessibility. Innovations in next-generation sequencing, digital pathology, point-of-care testing, artificial intelligence, liquid biopsy, automation, and telemedicine are driving market growth and transforming patient care. As these technologies continue to evolve and integrate into clinical practice, the European IVD market is poised for continued expansion, offering new opportunities for stakeholders and improving health outcomes across the continent.