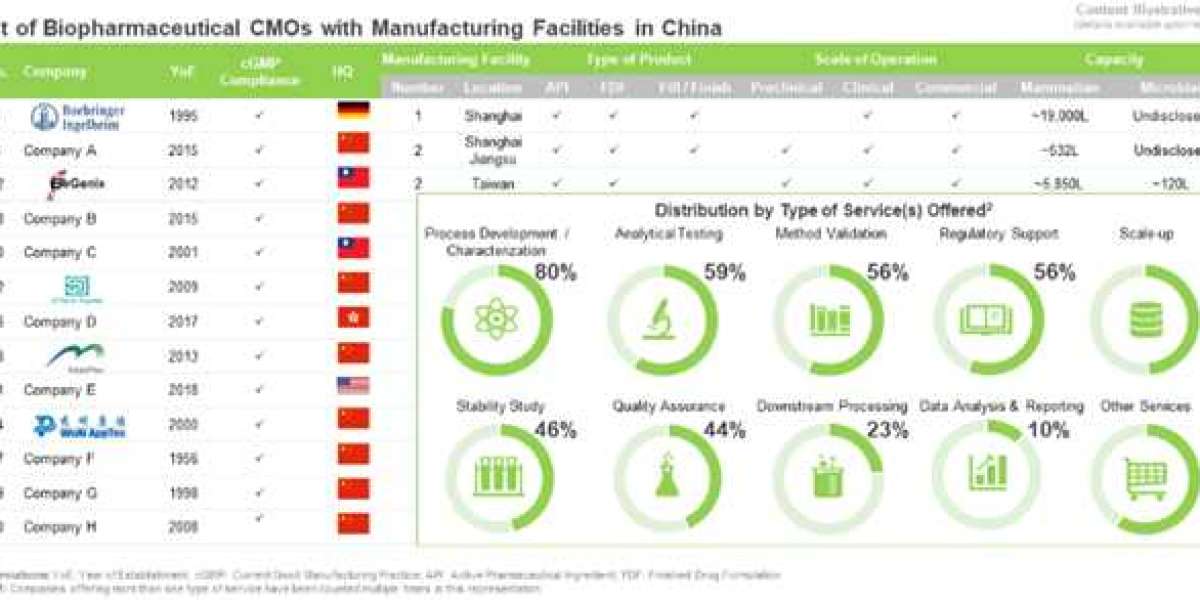
The current biopharmaceuticals market is characterized by high quality standards and a rapidly growing demand for novel and effective treatment options. It is, therefore, a technically challenging industry in which outsourcing has grown into a promising and prominent segment, especially for manufacturing and fill / finish operations. Outsourcing biopharmaceutical manufacturing operations to Asia is increasing at a rapid pace. In this, China has emerged as an attractive destination for outsourcing.
To request a sample report: https://www.rootsanalysis.com/reports/313/request-sample.html
Driven by rapid technological development, and an increasingly favorable policy landscape, the Chinese biopharmaceutical industry is projected to witness significant growth in the coming years. Considering the complications associated with the production and handling of biologic drugs, biopharmaceutical developers are always on the lookout for reliable contract service providers. As a result, CMOs / CDMOs in China have invested significant capital and other resources in order to develop the required scientific acumen, technical expertise and production capacity for biopharmaceuticals, over the past decade. As a result, several indigenous players currently claim to offer services and solutions at par with world-class biopharmaceutical CMOs and CDMOs. Further, several service provider companies based in developed regions, have also established wholly / partially owned facilities in this region. This strategic shift in location of manufacturing operations enables the aforementioned players to leverage the various region-specific advantages, related to biopharmaceutical manufacturing, which China has to offer.
To order customize report: https://www.rootsanalysis.com/reports/china-biopharmaceutical-contract-manufacturing/request-customization.html
Further, the recent approval of the market authorization holder (MAH) system on a national level, which was being run as a pilot program in several provinces since 2016, is a big achievement. This has led to an increase in outsourcing contracts to players based in China, which is further expected to decrease the idle capacity of CMOs, thereby reducing overall cost and increasing productivity; it is worth mentioning that, owing to rise in expansion initiatives carried out by biopharmaceutical CMOs in this region, over 50% of the biomanufacturing capacity is estimated to be idle / not utilized. In addition, as multiple biologics are likely to lose their patent exclusivity in the near future, the demand for biosimilars, especially monoclonal antibody (mAb) therapeutics, is expected to rise. Since these products cannot be marketed at exorbitant prices, firms often outsource their manufacturing operations to emerging regions, such as China, to enable savings; this is further likely to boost the growth of the Chinese biopharmaceutical CMOs market. Some the main advantages of outsourcing manufacturing operations to China-based CMOs / CDMOs are briefly described below:
- Availability of Skilled Labor: The country has a large pool of educated and capable individuals who are qualified to work in biopharmaceutical manufacturing plants. The recent increase in stringency of immigration policies, coupled to a scarcity of jobs in the developed regions, have cause many Chinese nationals to return to China. Such individuals, who have been trained in the West, are highly likely to be hired by Chinese firms. Further, a number of biopharmaceutical companies, such as Boehringer Ingelheim, GE Healthcare, Merck and Thermo Fisher Scientific, have either already established, or are planning to establish, manufacturing operations in China. This is anticipated to create several job opportunities for scientists and pharma workers in the region.
- Cost Advantages: Labor in developing regions, such as China, is much less expensive when compared to the US and Europe. This has garnered the interest of many multinational biopharmaceutical companies, especially those based in developed regions, causing them to offshore a lot of their production operations.
- Access to a Large Indigenous Marketplace: Product manufactured in China are granted easier access to the local market. On the other hand, regulations in China are actually prohibitive when it comes to foreign developers attempting to sell their offerings in the Chinese market. Considering the fact that the country is home to one of the largest populations, the region does offer a lucrative marketplace.
Evolving Regulatory Framework and Government Support: The Chinese government is very supportive of local economic development, offering subsidies and incentives to indigenous initiatives. The introduction of the MAH guidelines, which provided special allowances for approval of drugs manufactured by contract organizations offered a significant boost to the contract services industry in the region.
For more information, please click on the following link:
You may also be interested in the following reports:
- Peptide Therapeutics
- Biopharmaceutical Excipient Manufacturing Market
- Single-use Sensors for Bioprocessing Market
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/