Clinical Trial Outsourcing Market Methodology:
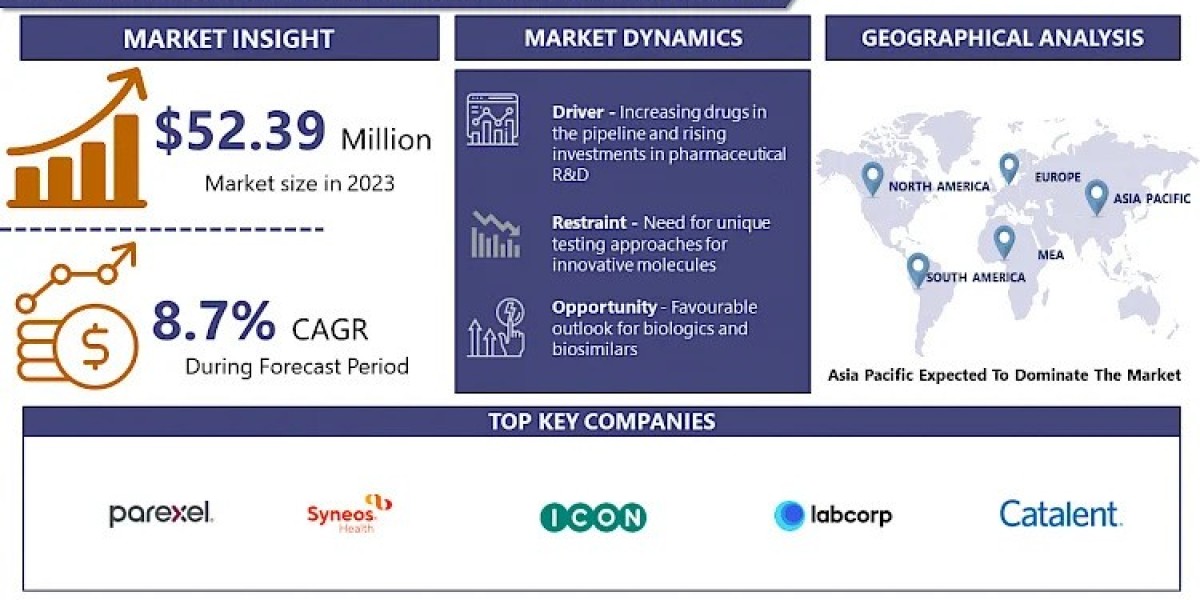
Global Clinical Trial Outsourcing Market Size Was Valued at USD 52.39 Billion in 2023, and is Projected to Reach USD 111.01 Billion by 2032, Growing at a CAGR of 8.7% From 2024-2032.
Introspective Market Research offers comprehensive Clinical Trial Outsourcing Market research studies, providing valuable insights and strategic guidance to businesses worldwide. We ensure reliability and accuracy in our reports for informed decision-making.
The keyword market research study ensures the highest level of accuracy and reliability as we precisely examine the overall industry, covering all the market fundamentals. By leveraging a wide range of primary and secondary sources, we establish a strong foundation for our findings. Industry-standard tools like Porter's Five Forces Analysis, SWOT Analysis, and Price Trend Analysis further enhance the comprehensiveness of our evaluation.
Our study also discusses the complete keyword market ecosystem, explaining the various market stakeholders, their functions and interdependencies between them. Further, with an emphasis on comprehensive segmentation analysis and geographical coverage, the study enables a profound understanding of regional trends. Moreover, we explore external factors providing a comprehensive view of the market dynamics.
Clinical Trial Outsourcing Market Dynamics:
The increasing complexity of clinical trials and the demand for quicker time-to-market for new drugs are driving the outsourcing of clinical trials. Pharmaceutical companies are increasingly depending on Contract Research Organizations (CROs) to manage trial logistics, such as patient recruitment and data management. Outsourcing aids in cutting operational expenses and optimizing procedures, particularly for smaller companies lacking robust in-house resources. Regulatory requirements and the opportunity to tap into worldwide patient populations are additional reasons why companies seek out partnerships with skilled CROs, who have the expertise to understand local regulations and streamline clinical trials in various regions.
Market Trends:
The clinical trial outsourcing sector is experiencing a change towards decentralized trials, facilitated by digital health tools such as telemedicine, remote monitoring, and wearable devices. This method lessens the load on patients and improves enrolment, especially for uncommon diseases or populations spread out geographically. There is an increasing emphasis on real-life data (RWE) and patient-focused trial designs. Moreover, collaborations between CROs and tech firms are increasing, with automation, AI, and big data analysis being incorporated into trial management to improve precision and efficiency.
Get an Inside Scoop of Study, Request now for Sample Study @:
https://introspectivemarketresearch.com/request/16737
Market Opportunities:
The growing complexity of drug development, especially in personalized medicine and biologics, offers substantial chances for outsourcing clinical trials. CROs can provide unique knowledge and technologies that smaller pharmaceutical companies may lack internally. In Asia-Pacific and Latin America, developing markets provide affordable trial locations with a variety of patient demographics. Furthermore, the emergence of decentralized and virtual clinical trials provides new opportunities for advancements in trial management, data gathering, and patient involvement, resulting in more adaptable, quicker, and patient-centric trials.
Clinical Trial Outsourcing Market Segmentation:
By Phase
- Phase I
- Phase II
- Phase III
- Phase II
- Phase IV
By Service Type
- Laboratory Services
- Bioanalytical Testing Services
- Decentralized Clinical Trial Services
- Analytical Testing Services
By Therapeutic Area
- Oncology
- Infectious Diseases
- Neurology
- Metabolic
- Disorders
- Immunology
By Application
- Small Molecules
- Monoclonal Antibodies
- Vaccine
- Cell & Gene Therapy.
Check for Best Quote @:
https://introspectivemarketresearch.com/request/16737
Clinical Trial Outsourcing Market Key Players:
- CHARLES RIVER LABORATORIES (US)
- CATALENT (US)
- EMMES COMPANY (US)
- SYNEOS HEALTH (US)
- FORTREA INC. (US)
- ADVANCED CLINICAL (US)
- THERMO FISHER SCIENTIFIC INC. (US)
- FRONTAGE LABS (US)
- ACM GLOBAL LABORATORIES (US)
- WORLDWIDE CLINICAL TRIALS (US)
- CTI CLINICAL TRIAL & CONSULTING (US)
- FIRMA CLINICAL RESEARCH (US)
- CELERION (US)
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH (EUROPE)
- PHARMASERV INTERNATIONAL (GERMANY)
- APTIV SOLUTIONS (FRANCE)
- DOVE QUALITY SOLUTIONS (UK)
- CLINIGEN GROUP (UK), and other key players.
Clinical Trial Outsourcing Market Regional Analysis:
The United States, in particular, leads in clinical research and outsourcing activities, attracting a significant portion of global clinical trials across various therapeutic areas. It boasts a wealth of expertise in advanced technologies, a large pool of skilled professionals, and a conducive environment for conducting trials efficiently.
Get Detailed Overview of Report @
https://introspectivemarketresearch.com/request/16737
Company Profiles and Competitive Analysis:
COMPANY PROFILES AND COMPETITIVE ANALYSIS
- COMPETITIVE LANDSCAPE
- Competitive Positioning
- Clinical Trial Outsourcing Market Share by Manufacturer (2024)
- Industry BCG Matrix
- Heat Map Analysis
- Mergers & Acquisitions
- ARIEL CORPORATION
- Company Overview
- Key Executives
- Company Snapshot
- Role of the Company in the Market
- Sustainability and Social Responsibility
- Operating Business Segments
- Product Portfolio
- Business Performance (Production Volume, Sales Volume, Sales Margin, Production Capacity, Capacity Utilization Rate)
- Key Strategic Moves and Recent Developments
- SWOT Analysis
Related Reports:
Minimally Invasive Surgery Market
https://introspectivemarketresearch.com/reports/minimally-invasive-surgery-market/
Hip Replacement Market
https://introspectivemarketresearch.com/reports/hip-replacement-market/
About US:
We are technocratic market research and consulting company that provides comprehensive and data-driven market insights. We hold the expertise in demand analysis and estimation of multidomain industries with encyclopaedic competitive and landscape analysis. Also, our in-depth macro-economic analysis gives a bird’s eye view of a market to our esteemed client.
Our team at Introspective Market Research focuses on result-oriented methodologies which are based on historic and present data to produce authentic foretelling about the industry. Introspective Market Research’s extensive studies help our clients to make righteous decisions that make a positive impact on their business. Our customer-oriented business model firmly follows satisfactory service through which our brand name is recognized in the market.
Contact US:
Canada Office
Introspective Market Research Private Limited, 138 Downes Street Unit 6203- M5E 0E4, Toronto, Canada.
APAC Office
Introspective Market Research Private Limited, Office No. 401, Saudamini Commercial Complex, Chandani Chowk, Kothrud, Pune India 411038
Ph no: +1-773-382-1049