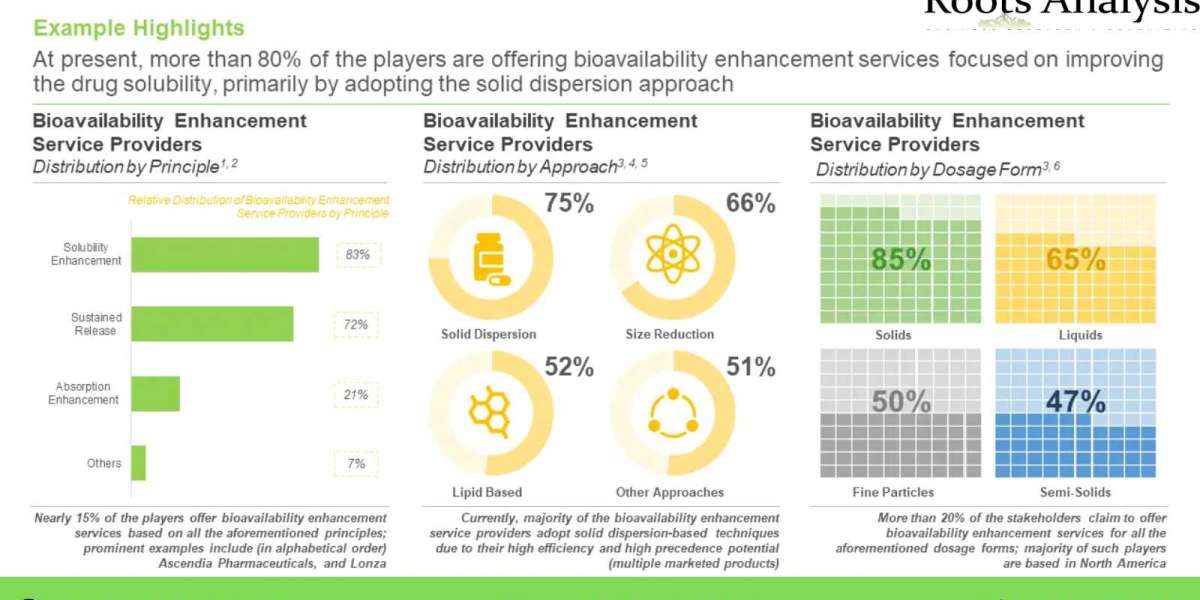
Bioavailability refers to the proportion of a drug that reaches systemic circulation unaltered, post dose administration. Additionally, it is a direct consequence of the absorption potential of an active pharmaceutical ingredient (API). The bioavailability of a drug is dependent on several factors, such as solubility, route of administration and metabolism of the drug post administration. The following figure provides different factors that have been reported to impact drug bioavailability either directly or indirectly. For this purpose, substantial mergers and acquisitions have been reported in this market, as service providers strive to become one-stop-shops, to cater to the diverse needs for their clientele. In addition, several stakeholders are engaged in the development of proprietary technologies, based on sustained release principle and bioavailability enhancers, to maintain a competitive edge in this rapidly emerging market. An increasing number of drug candidates have been granted approval via the 505(b)(2) pathway; the aforementioned pathway is used to gain approval for novel formulations consisting of previously approved active pharmaceutical ingredient (API). Additionally, given the shifting focus of drug developers towards development of lipophilic drug compounds, the industry is actively undertaking efforts to identify various bioavailability enhancement techniques, in order to mitigate the challenge of low bioavailability and stability.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/198/request-sample.html
- Solubility: The solubility of a substance refers to its ability to dissolve in a solvent and form a homogeneous system. Drug solubility is considered to be one of the most important factors responsible for enabling a therapeutic intervention to be efficiently distributed via systemic circulation. It is worth highlighting that a drug’s solubility is directly proportional to its bioavailability and hence, is one of the primary parameters that is modified during bioavailability enhancement
- Permeability: Permeability refers to the ability of a molecule to pass through biological membranes. Similar to solubility, drug permeability plays a key role in its absorption and systemic distribution. Membrane based efflux mechanisms and other structural barriers are the major biological elements responsible for poor permeability of drugs. Permeability of a drug is also proportional to its bioavailability; however, it is possible to improve this characteristic by modifying the physiochemical properties of the drug to render them more suitable for absorption across different types of biological membranes.
- Molecule Size: Rate of absorption increases inversely with the size of a drug molecule. However, several studies have demonstrated that, absorption mechanisms vary significantly for different sizes of molecules. For instance, larger molecules get absorbed either by endocytosis or facilitated diffusion, whereas smaller molecules are absorbed via aqueous diffusion or through lipid channels.
- Degree of Ionization: Degree of ionization has a direct impact on drug absorption. Higher degree of ionization of a drug molecule results in poor solubility. As a general rule, basic drugs get ionized in acidic medium and are therefore, poorly soluble in acidic medium. The same rule applies to acidic drugs in basic medium.
- Formulation: The formulation process involves the addition of certain excipients, which are inactive substances that augment the properties of the drugs. Despite being inactive, these substances can influence drug bioavailability.
- Concentration: According to Fick’s law of diffusion, higher drug concentration in a tissue microenvironment, leads to higher flux through the membrane, resulting in a high rate of absorption.
- Dosage Forms: Drugs can be administered into the body via a number of routes, such as oral, transdermal, ocular and intramuscular. However, it is important to note that the mode of administration plays a very significant role in drug bioavailability. Several studies have demonstrated that only intravenous injections offer 100% drug availability in systemic circulation compared to any other route.
- Physical Forms: The physical form of the drug (solid, liquid or inhalable powder) also affects its rate of absorption. It is worth highlighting that drugs get more absorbed in gaseous form followed by liquid and solid form respectively.
- Chemical Nature: The chemical make-up of a drug is also an important determinant of its absorption potential and bioavailability. It has been observed that inorganic salts are more readily absorbed than organic compounds, when administered via the oral route.
Further, given the rapid increase in the number of NCEs with low solubility and considering that this trend persists in the future as well, there is likely to be a surge in the demand for technologies that facilitate bioavailability enhancement. There are a number of technologies which are already available for improving drug bioavailability. Broadly these technologies can be distributed across three main categories, namely physical, chemical and biological approaches. However, as pharmacological interventions gradually become more complex and novel drug classes are introduced, further innovation may be required in the development of better bioavailability enhancement techniques / methods.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/bioavailability-enhancement-technologies-and-services/198.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- RNAi Market: Therapeutics and Technologies (Focus on siRNA, miRNA, shRNA and DNA) (3rd Edition): Industry Trends and Global Forecasts, 2022-2035
- Global Therapeutic Vaccines Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415