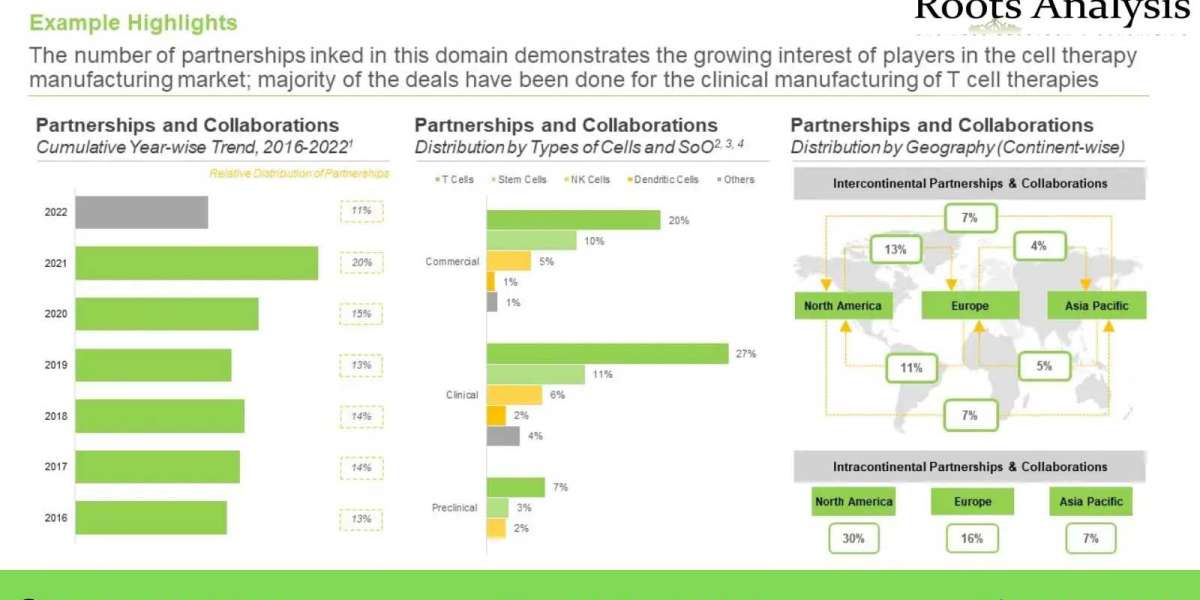
Considering the vast potential of cellular therapies in the treatment of rare disorders and sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, cell therapies have garnered considerable attention of players engaged in the healthcare industry, in the past few years.
The focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process. It is important to mention that we have forecasted the evolution of the overall cell therapy manufacturing market, focusing on T-cell immunotherapies, dendritic cell therapies, NK cell therapies and stem cell therapies. Considering the sufficient body of evidence validating the clinical benefits / therapeutic potential of this complex class of biologic drugs, the focus of stakeholders has now shifted to optimizing the cell therapy manufacturing process.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/285/request-sample.html
One such emerging concept, namely GMP-In-A Box, offers several advantages, including increased throughput, decreased idle time between batch runs and reduced manual labor. However, the delicate nature of steps involved in the cell therapy production process is known to hinder the overall automation process. Further, the lack of specialized infrastructure and limited expertise available in this domain are some of the known challenges impacting the growth of this segment.
Despite the challenges associated with the development and production of such therapies, we believe, that the benefits offered by them outweigh the hurdles. They are likely to serve as important drivers of the industry’s growth. Efforts to introduce automation technologies in cell therapy manufacturing are underway, and if implemented successfully, can significantly help in the elimination of human intervention and reduce the risk of contamination. As a consequence, it is likely to result in a marked increase in product consistency, ensure the maintenance of sterility, and decrease the production time and cost. In a nutshell, cell therapies are expected to soon represent one of the prominent therapeutic options within the mainstream healthcare.
For additional details, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Targeted protein degradation market, 2022-2035
- Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091