Global Point of Care Molecular Diagnostics Market Statistics: USD 8.7 Billion Value by 2033
Summary:
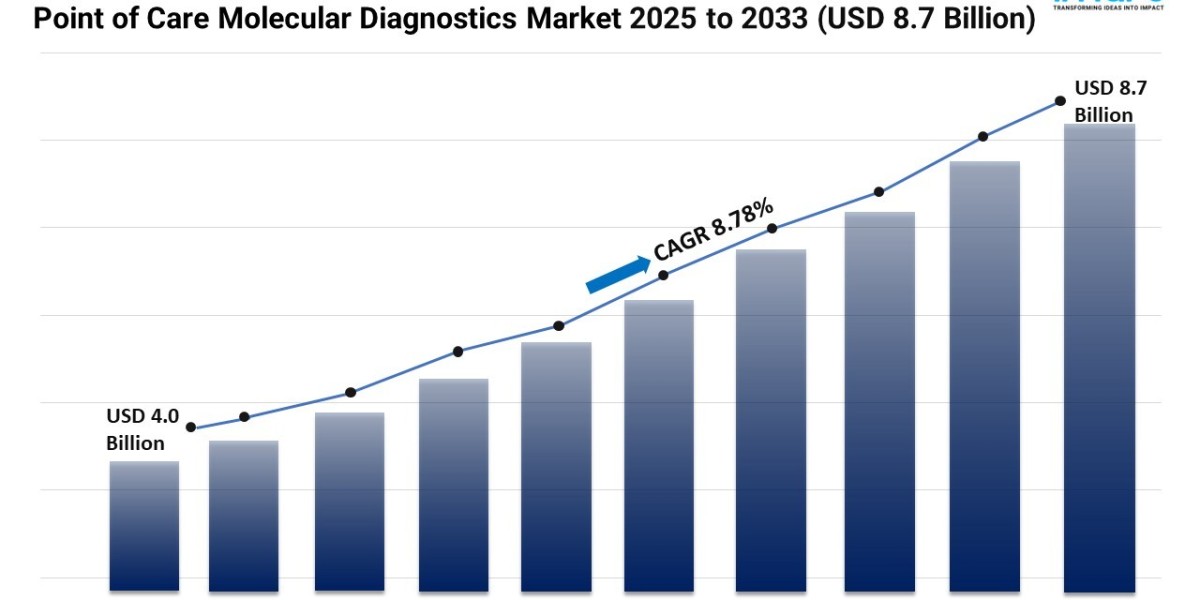
- The global point of care molecular diagnostics market size reached USD 4.0 Billion in 2024.
- The market is expected to reach USD 8.7 Billion by 2033, exhibiting a growth rate (CAGR) of 8.78% during 2025-2033.
- North America leads the market, accounting for the largest point of care molecular diagnostics market share.
- Assays and kits exhibit a clear dominance in the market due to their essential role in facilitating rapid, accurate, and easy-to-use testing in POC settings.
- Polymerase chain reaction (PCR) represents the largest segment because of its high sensitivity, specificity, and widespread use in detecting a broad range of pathogens in POC diagnostics.
- Wet anaerobic digestion remains a dominant segment in the market, allowing for better methane production and more consistent energy generation.
- The rising incidence of infectious diseases globally is driving the need for point of care (POC) diagnostic services.
- Ongoing advancements, particularly in the miniaturization of molecular diagnostic devices, are propelling the growth of the market.
Industry Trends and Drivers:
- Increasing Prevalence of Infectious Diseases:
The rising incidence of infectious diseases globally is driving the need for point of care (POC) diagnostic services. Infectious diseases like influenza and tuberculosis require rapid diagnosis for timely treatment, which is catalyzing the demand for POC testing. Unlike traditional laboratory methods, POC molecular diagnostics provide results quickly, often within an hour, allowing for immediate clinical decisions. This speed is crucial in controlling outbreaks and improving patient outcomes. Additionally, the ability to conduct tests at or near the site of patient care reduces the need for specialized laboratory infrastructure, making it easier to manage infectious diseases in remote or resource-limited settings. As a result, healthcare providers are increasingly adopting POC molecular diagnostics as part of their diagnostic strategies.
- Technological Advancements and Miniaturization:
Ongoing advancements, particularly in the miniaturization of molecular diagnostic devices, are propelling the growth of the market. Innovations in microfluidics, lab-on-a-chip technologies, and portable Polymerase Chain Reaction (PCR) devices are enabling the development of compact and user-friendly POC diagnostic tools. These devices can perform complex molecular tests with high accuracy and sensitivity, rivaling traditional laboratory equipment. The ease of use and portability of these devices allow for decentralized testing, where diagnostics can be conducted at the bedside of the patient, in clinics, or even in non-medical settings. This technological progress is expanding the application of molecular diagnostics beyond specialized labs, making it more accessible and practical for widespread use.
- Growing Demand for Personalized Medicine:
The increasing emphasis on personalized medicine is supporting the market growth. Personalized medicine tailors treatment plans based on the genetic makeup and specific characteristics of the disease, requiring precise and rapid diagnostic tools. POC molecular diagnostics provide the ability to quickly identify genetic markers, pathogens, and mutations that inform personalized treatment strategies. For instance, in oncology, POC molecular tests can detect specific cancer mutations, enabling oncologists to choose the most effective targeted therapies. This tailored approach not only improves treatment efficacy but also lowers the risk of adverse complications, leading to better patient outcomes. The growing focus on personalized healthcare, combined with the need for timely and accurate diagnostics, is driving the demand for POC molecular diagnostics.
Request for a sample copy of this report: https://www.imarcgroup.com/point-of-care-molecular-diagnostics-market/requestsample
Point of Care Molecular Diagnostics Market Report Segmentation:
By Product and Services:
- Assays and Kits
- Instruments and Analyzers
- Software and Services
Assays and kits exhibit a clear dominance in the market due to their essential role in facilitating rapid, accurate, and easy-to-use testing in POC settings.
By Technology:
- Polymerase Chain Reaction (PCR)
- Hybridization
- DNA sequencing
- Microarray
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Others
Polymerase chain reaction (PCR) represents the largest segment because of its high sensitivity, specificity, and widespread use in detecting a broad range of pathogens in POC diagnostics.
By Application:

- Infectious Diseases
- Oncology
- Hematology
- Prenatal Testing
- Endocrinology
- Others
Infectious diseases hold the biggest market share as POC molecular diagnostics are crucial for the timely detection and management of these diseases, particularly in outbreak scenarios.
By End User:
- Physicians’ Offices
- Hospitals and ICUs
- Research Institutes
- Others
On the basis of the end user, the market has been segregated into physicians’ offices, hospitals and ICUs, research institutes, and others.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America dominates the market attributed to its advanced healthcare infrastructure, high adoption rates of new technologies, and strong presence of key industry players.
Top Point of Care Molecular Diagnostics Market Leaders:
The point of care molecular diagnostics market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- Abbott Laboratories
- Becton Dickinson and Company
- Binx Health Inc.
- Co-Diagnostics Inc.
- F. Hoffmann-La Roche Ltd
- Gene STAT Molecular Diagnostics LLC
- Hemocue AB (Danaher Corporation)
- Meridian Bioscience Inc.
- Molbio Diagnostics Private Limited
- Quidel Corporation
- Siemens Healthcare GmbH (Siemens AG)
- Thermo Fisher Scientific Inc.
- Visby Medical Inc.
Note: If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1–631–791–1145