Esketamine Market Overview (2024-2032)
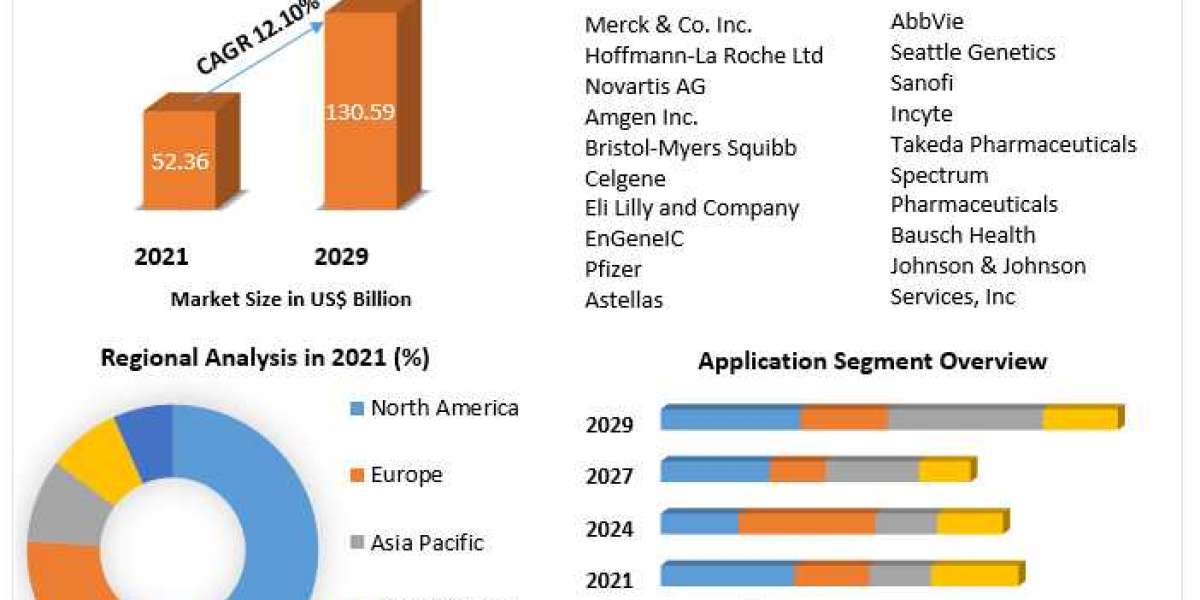
The esketamine market is expected to experience substantial growth from 2024 to 2032, driven by the increasing prevalence of treatment-resistant depression, the rising recognition of the drug's efficacy in treating mental health disorders, and the growing adoption of innovative therapeutic options. Esketamine, a nasal spray formulation of ketamine, has emerged as a critical advancement in the mental health field, particularly for patients who have not responded to traditional antidepressants. As the global burden of mental health disorders continues to rise, esketamine is gaining prominence as a novel treatment option, further supported by regulatory approvals in several countries.
Esketamine was first approved by the U.S. FDA in 2019 for use in treatment-resistant depression (TRD) under the brand name Spravato. Its approval marked a significant milestone in the treatment of mental health disorders, as esketamine offers a unique mechanism of action compared to traditional antidepressants. Unlike conventional antidepressants that target serotonin, norepinephrine, and dopamine, esketamine works by targeting the NMDA receptors in the brain, potentially providing faster relief for patients suffering from severe depression. The drug’s rapid onset of action and potential to improve the quality of life for patients who have exhausted other treatment options make it a valuable addition to the antidepressant landscape.
Key Regions and Countries
The esketamine market is influenced by the healthcare infrastructure, regulatory approval processes, and the prevalence of treatment-resistant depression in various regions. The market dynamics in North America, Europe, and Asia-Pacific, particularly, are key to understanding the overall growth trajectory of this therapeutic sector.
North America
North America holds the largest share of the esketamine market, particularly in the United States. The FDA’s approval of Spravato for treatment-resistant depression has significantly propelled market growth in this region. Additionally, the widespread adoption of esketamine treatment in U.S. healthcare settings, coupled with increasing healthcare spending and an aging population, is expected to drive continued demand for the drug. Esketamine is also being used in clinical settings for other mental health disorders, which further expands its potential market. The integration of esketamine into outpatient clinics and its reimbursement by insurance providers have contributed to its increasing accessibility in the U.S.
In Canada, the uptake of esketamine has also been strong, thanks to favorable regulatory frameworks and the country’s focus on improving mental health care. As mental health issues become a priority for healthcare policymakers in North America, the market for esketamine is poised for steady growth.
Europe
Europe represents another significant market for esketamine, with countries like Germany, the UK, and France leading the way in the adoption of the treatment. European regulators, including the European Medicines Agency (EMA), approved esketamine for use in treatment-resistant depression, and several countries have included it in their mental health care programs. The European market for esketamine is growing as the drug gains traction in both public and private healthcare systems. Countries such as Germany and the UK have robust mental health infrastructures that are conducive to the adoption of new treatments like esketamine, and public health initiatives aimed at improving access to mental health care further support its uptake.
The increasing burden of mental health disorders in Europe, coupled with high awareness of treatment-resistant depression, will likely drive the demand for esketamine in the coming years. As more healthcare providers implement esketamine into their treatment regimens, the market is expected to expand.
Asia-Pacific
The Asia-Pacific region is emerging as a key market for esketamine, with countries like Japan, China, and India seeing growing adoption. Mental health care awareness is increasing in many parts of Asia-Pacific, and countries like Japan are placing greater emphasis on addressing depression and other mental health conditions. However, the market in this region faces challenges such as limited healthcare infrastructure in some countries and cultural barriers related to mental health treatment. Despite these challenges, the prevalence of mental health disorders, especially treatment-resistant depression, is growing, and esketamine’s ability to address this unmet need has fueled interest in its use.
In India and China, the increasing number of patients suffering from depression, combined with expanding access to healthcare services, provides a promising growth opportunity for esketamine. However, the cost of treatment and regulatory hurdles may slow its widespread adoption in the region.
Latin America and Middle East & Africa
In Latin America and the Middle East & Africa, the esketamine market is in the early stages of growth. Although the prevalence of mental health disorders is on the rise, there are significant challenges in terms of access to advanced treatments like esketamine. In many parts of these regions, healthcare systems are still developing, and access to novel treatments may be limited. However, increasing awareness of mental health issues and ongoing efforts to improve healthcare services present opportunities for market growth. As regulatory frameworks evolve and mental health care becomes a priority, the adoption of esketamine is expected to increase gradually in these regions.
Research Methodology
The research methodology for analyzing the esketamine market involves both primary and secondary research approaches. Primary research includes interviews and surveys with healthcare professionals, mental health experts, and pharmaceutical industry representatives. These interactions help gather insights into the current treatment landscape for treatment-resistant depression, the adoption of esketamine, and its potential to address unmet medical needs.
Secondary research involves reviewing published market reports, scientific literature, clinical trials, and regulatory updates from key health authorities such as the U.S. FDA, EMA, and global health organizations. This provides a comprehensive understanding of the market dynamics, competitive landscape, and the current regulatory environment for esketamine.
Market Dynamics
Several factors are driving the growth of the esketamine market, including increasing mental health awareness, the unmet need for effective treatments for treatment-resistant depression, and the drug’s unique mechanism of action. At the same time, challenges such as cost and accessibility remain key considerations.
Drivers:
- Increasing Prevalence of Mental Health Disorders: The global rise in depression, especially treatment-resistant depression, is a key driver for the demand for esketamine. As traditional antidepressants fail to work for a significant portion of patients, esketamine provides an alternative with a faster onset of action.
- Regulatory Approvals and Market Access: The approval of esketamine in major markets such as the U.S., Europe, and Japan has opened up significant opportunities. As more countries approve the drug, its market potential expands.
- Rapid Onset and Efficacy: Esketamine’s rapid onset of action and efficacy in patients who have not responded to traditional antidepressants give it a competitive edge over conventional treatments. Its ability to provide quick relief is a major selling point.
Challenges:
- High Treatment Costs: Esketamine’s high cost is a significant barrier to widespread adoption, particularly in low-income countries. Patients may face challenges in accessing the drug, and insurers may limit reimbursement for its use.
- Side Effects and Safety Concerns: Esketamine, like other ketamine derivatives, has been associated with side effects such as dissociation, increased blood pressure, and potential misuse. These concerns may impact its broader adoption.
- Healthcare Infrastructure: In developing regions, limited healthcare infrastructure and the need for professional supervision during esketamine administration can hinder its accessibility.
Key Questions Answered
What are the key factors driving the growth of the esketamine market?
- The increasing prevalence of mental health disorders, especially treatment-resistant depression, regulatory approvals, and the drug’s rapid onset of action are key growth drivers.
Which regions are expected to see the most significant growth in the esketamine market?
- North America, Europe, and Asia-Pacific are expected to experience significant growth, with North America leading in market share due to the widespread adoption of esketamine.
What are the challenges facing the market?
- Challenges include the high cost of treatment, side effects, safety concerns, and limited healthcare infrastructure in certain regions.
How can pharmaceutical companies address these challenges?
- Companies can focus on improving access through partnerships with healthcare providers, reducing treatment costs, and conducting further research to address safety concerns.
Reasons to Buy
- Growing Demand: The market is expected to grow due to the increasing prevalence of mental health disorders and the unmet need for effective treatments.
- Regulatory Support: Esketamine has received approval in key regions, which will drive adoption and market growth.
- Innovative Treatment: Esketamine’s unique mechanism of action and fast-acting nature make it a promising treatment option for patients with treatment-resistant depression.
In conclusion, the esketamine market is poised for substantial growth, driven by increasing mental health awareness, unmet medical needs, and regulatory support. As more countries approve esketamine for use and healthcare providers embrace it as a viable treatment option, the market will continue to expand, offering significant opportunities for stakeholders in the pharmaceutical and healthcare industries.