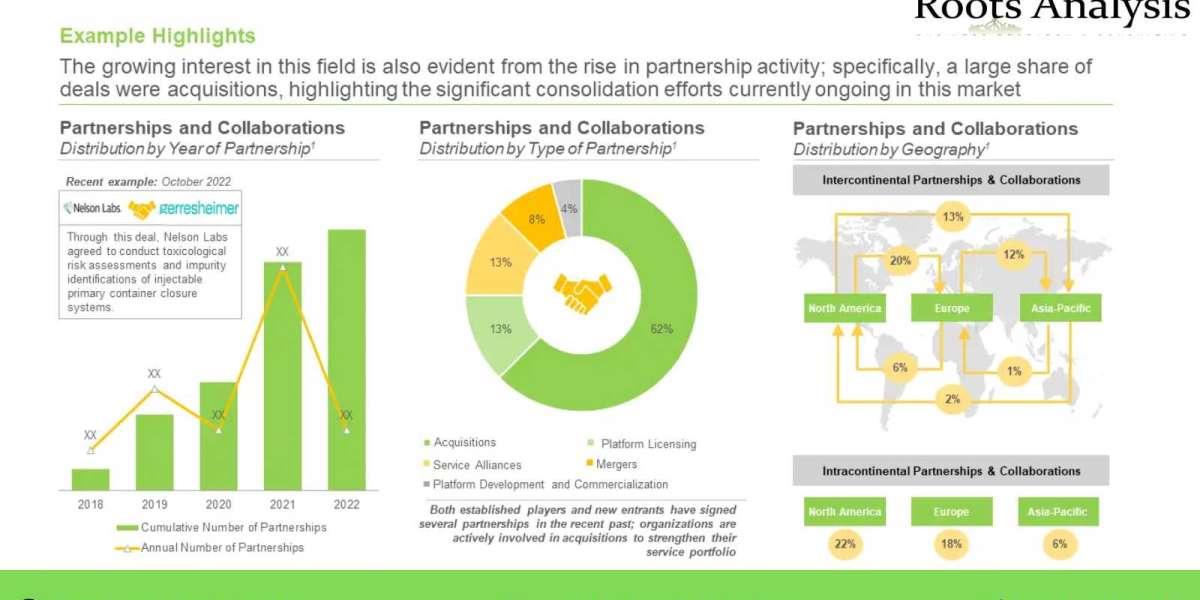
Further, researchers have been able to link the mutations caused by genetic toxicology to various disease indications, including several oncological and genetic disorders. In addition, it is believed that the changes introduced in the genetic material can be passed down to future cell generations. Similarly, mutagenicity refers to the introduction of perpetual transmissible changes in the genomic structure of organisms, enabling mutations in cells. Therefore, testing of genotoxicity / mutagenicity is an essential component of the safety assessment of products, with the objective of preventing certain particles from negatively impacting the human health.
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/genotoxicity-testing-services-market/request-sample.html
Testing techniques used for genotoxicity / mutagenicity testing:
- Ames test: It is used to evaluate an agent’s mutagenic potential by reversing mutations in the tester mutant bacteria (E. coli, Salmonella typhi), as well as its ability to synthesize an essential amino acid required for growth.
- Mouse Lymphoma Assay / Hypoxanthine Guanine Phosphoribosyl Transferase: Mouse Lymphoma Assay is employed to assess an agent’s genotoxic potential. It determines the genetic variations affecting the expression of the thymidine kinase gene located on chromosome.
- Mutation Assay: Transgenic rodent somatic and germ cell gene mutation assay specifies an in vivo assay that perceives gene mutation causing agents. Transgenic mice or rats with several copies of chromosomally integrated plasmids or phage shuttle vectors are employed in this test.
- Comet Assay: The comet assay, also known as single cell gel electrophoresis (SCGE), is an efficient technique for identifying DNA strand breaks in a cell. It is used in molecular epidemiology, genotoxicity testing, and basic studies on DNA damage and repair.
- Chromosomal Aberration Test: The test is used to evaluate a substance's possible genotoxic risk. Mammalian cells are cultivated in vitro, exposed to a test substance, extracted, and then the frequency of structural asymmetry of the chromosomes is determined.
- Micronucleus Test: The micronucleus identifies chemicals (liquid or solid) that lead to cytogenetic damage, resulting in the formation of micronuclei containing complete or lagging chromosomal segments.
Hence, it can be concluded that players offering services for genotoxicity / mutagenicity testing have now become an integral part of various industries. Furthermore, considering the various active initiatives being undertaken by players based in this domain, we are led to believe that the opportunity for stakeholders in this industry is likely to grow at an exponential pace in the foreseen future.
For additional details, please visit
https://www.rootsanalysis.com/reports/genotoxicity-testing-services-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com